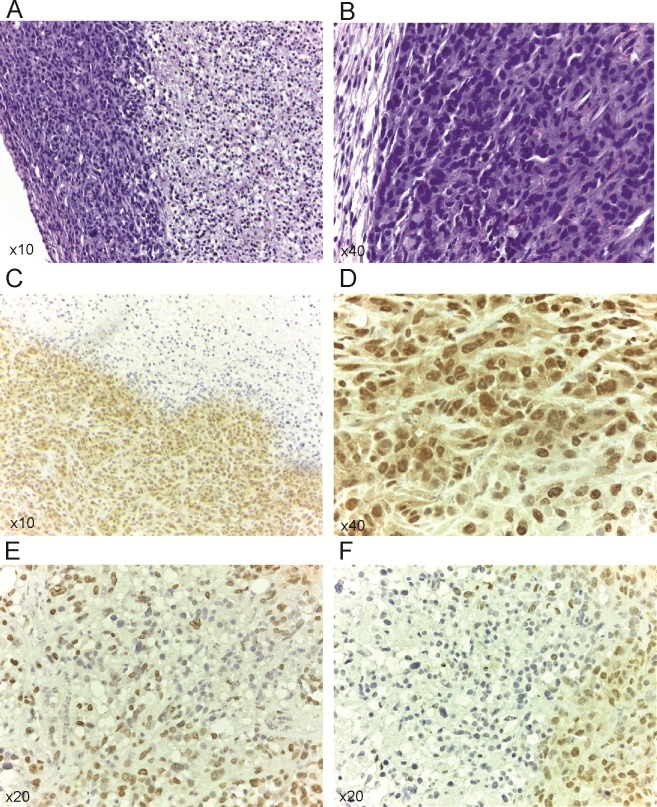
Fig 7. Expression of p-Met[Y1003] in control and in treated groups.
(A) Tumor section showing peripheral areas with preserved morphology and vast areas of central necrosis. H&E stain. (B) The tumor cells show high pleomorphism, hyperchromasia and increased mitotic activity. H&E stain (C) Immunohistochemistry for p-Met[Y1003]. Untreated controls demonstrate uniform intense immunopositivity in the peripheral vital areas of the tumor (lower left). The necrotic central parts of the tumor are immunonegative (upper right). (D) Untreated controls reveal intense immunopositivity in both nuclear and cytoplasmic distribution. (E, F) UFT+CTX treatment group shows significantly decreased staining intensity with only focal immunopositivity p-Met[Y1003] in a predominantly nuclear pattern. Vast areas of viable tumour are not staining (immunonegative).