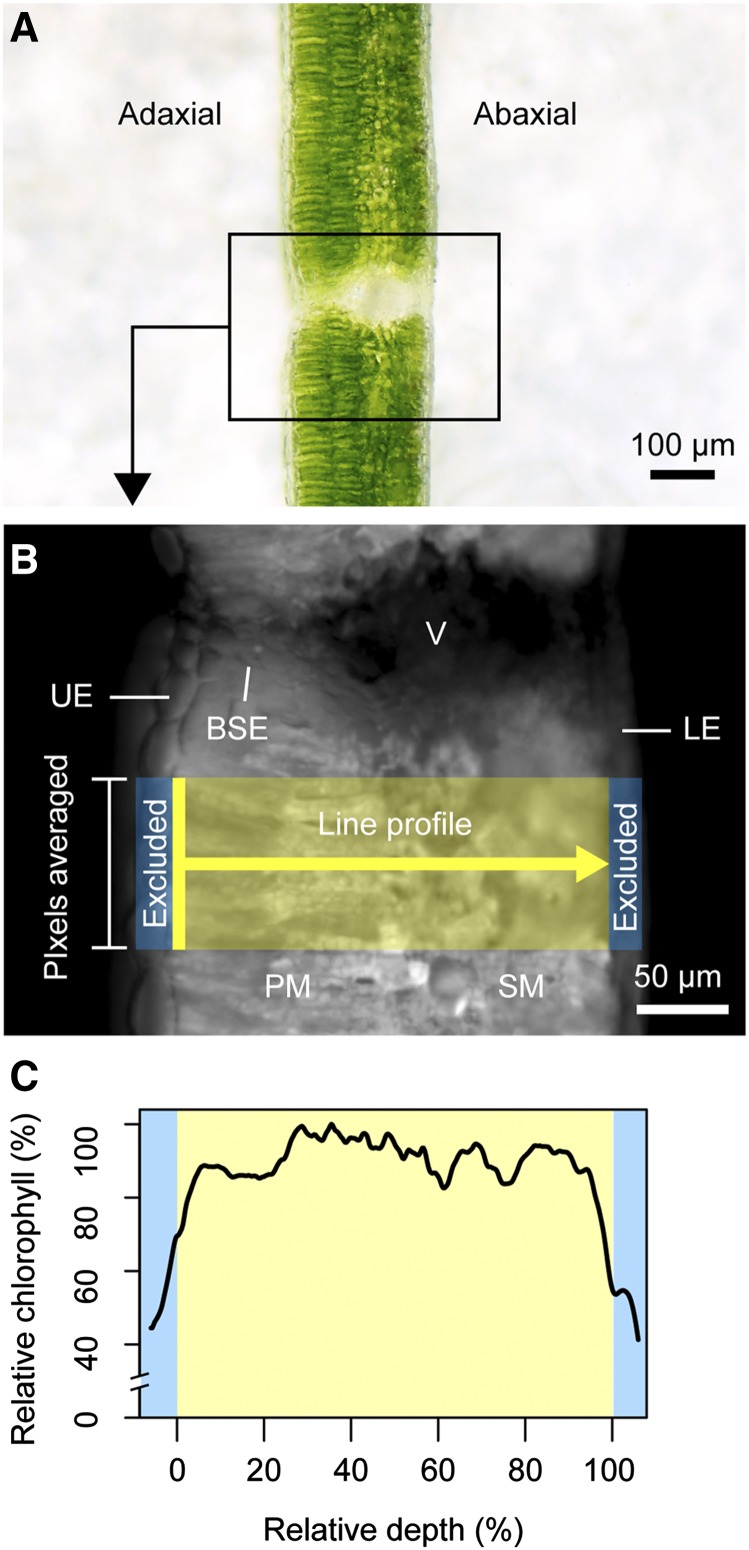
Figure 1.
Overview of the epi-illumination method. In brief, leaf samples are dark-adapted for ∼20 min before transverse sections (A) are cut and placed on damp filter paper for imaging. Samples are irradiated (<30 s), and fluorescence from chlorophyll is detected with a CCD camera. Chlorophyll content is proportional to pixel intensity in the resulting grayscale images (B; Vogelmann and Evans, 2002). The 1D chlorophyll distribution is measured in ImageJ using a line profile (line width mean of ∼100 pixels, or ∼60 µm at 20× magnification) drawn through the photosynthetic tissue. Nonphotosynthetic features such as veins (V), bundle sheath extensions (BSE), and upper epidermal (UE) and lower epidermal (LE) cells are excluded from the measurements. The profile begins at the upper edge of the mesophyll (e.g. palisade mesophyll if present; PM) and ends at the lower edge of the spongy mesophyll (SM). Previous studies using this technique included the epidermal cell layer (shown in C; blue bars), potentially making chlorophyll profiles look more bell-shaped than with this method. Chlorophyll profiles are normalized as a function of maximum intensity to allow for comparison (absolute fluorescence intensity varies across samples because of slight differences in exposure parameters and signal decay over time) and are represented as a function of relative depth (C; 0%, upper edge of mesophyll; 100%, lower edge of mesophyll).