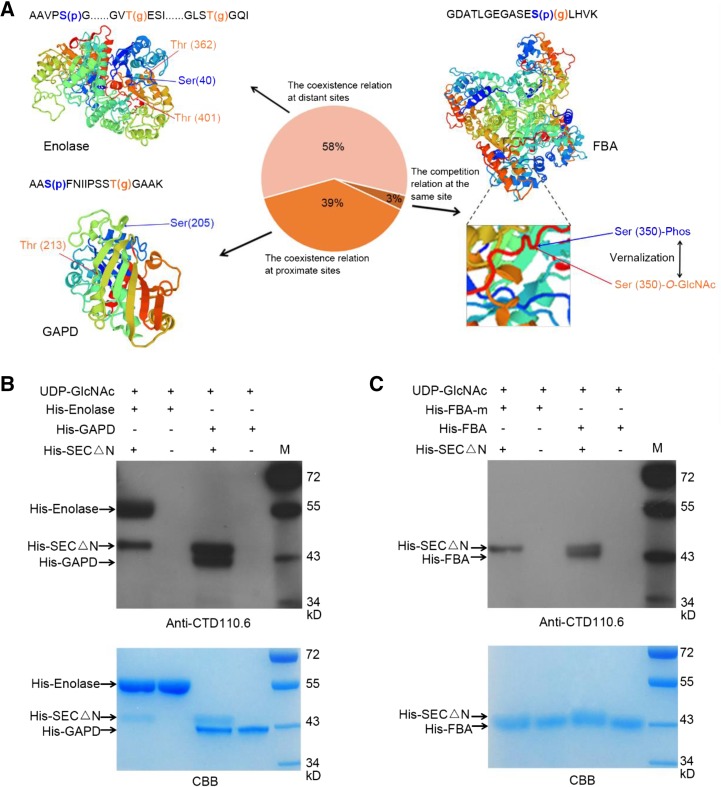
Figure 4.
Occupancy patterns between phosphorylation and O-GlcNAcylation modification in response to vernalization and SEC O-GlcNAcylates GAPD, Enolase, and FBA in vitro. A, Three-dimensional structures of the proteins (such as Enolase, GAPD, and FBA) with O-GlcNAcylation and phosphorylation modification predicted by Swiss-model (https://swissmodel.expasy.org/) and the sequences of identified peptides. The amino acid with O-GlcNAc modification (g) was orange, and the one with phosphorylation modification (p) was blue. There were two states between the O-GlcNAcylation and phosphorylation modifications from the results. One was the competition relation at the same site (3%), and the other was the coexistence relationship of the two modifications at proximate sites (58%) or distant sites (39%). B, Detection of O-GlcNAc modification of His-GAPD and His-Enolase, catalyzed by His-SECΔN in vitro. His-GAPD and His-Enolase were recombinantly expressed and affinity purified separately; His-SECΔN (expressing residues 801–1,062 of the C terminus) exhibited OGT activity [23]. O-GlcNAcylation of His-GAPD and His-Enolase were detected by anti-CTD110.6 antibody. C, Detection of O-GlcNAc modification of His-FBA and His-FBA-m catalyzed by His-SEC△N in vitro. His-FBA-m means the mutation of the three identified O-GlcNAcylated sites (T35, T320, and S350) of FBA.