Abstract
Objective:
Despite high risk for serious non-AIDS events (SNAEs) and accelerated age-related increases in inflammatory markers relative to HIV+ men, HIV+ women have been understudied, particularly in terms of stress impacts on immune parameters. The purpose of this study was to examine sex differences in glucocorticoid-immune stress response in mid-life HIV+ individuals, as poor glucocorticoid control of stress-induced inflammation may contribute to health risk in HIV+ women.
Methods:
Male and female participants completed a threat of shock laboratory stressor. Serum cortisol and cytokines (interleukin (IL)-6, IL-8, IL-10, IL-1β, C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interferon (IFN)- γ) were assessed at six timepoints prior to and in response to the stressor.
Results:
Participants included 8 HIV- controls (n=5 female) and 9 HIV+ (n=5 female) who were virally suppressed. Repeated measures mixed models revealed a significant sex by HIV status by time interaction for IL-10, IL-1ß, TNF-α, and cortisol. IL-10 response, an anti-inflammatory cytokine, was larger in males than females, regardless of HIV status. TNF-α response was blunted in HIV+ individuals compared with HIV-, and specifically in HIV+ women, IL-1ß and cortisol response were blunted.
Conclusions:
Individuals living with HIV may have impaired coordination between the immune system and hypothalamic pituitary adrenal (HPA) axis. HIV+ women in particular exhibited dysregulated IL-1ß and cortisol response to acute stress. Future work should focus on relationships among proinflammatory cytokines, stress, and SNAEs in HIV, with attention to sex as a biological variable.
Keywords: hypothalamic pituitary adrenal (HPA) axis, cytokine, psychosocial stress, sex as a biological variable, psychoneuroimmunology
BACKGROUND
Over 30 million individuals worldwide are living with human immunodeficiency virus (HIV), including approximately 17.8 million women (World Health Organization, 2017). With advances in HIV related medical care, HIV+ individuals are surviving into later adulthood and experiencing aging along with the general population. With longer lifespans comes increased exposure to the elevated inflammatory processes and chronic immune dysregulation that are common in HIV and contribute to increased risk for serious non-AIDS events (SNAEs) including cardiovascular disease (CVD), cancer, and HIV-1 associated neurocognitive disorder (HAND) (Hong & Banks, 2015). Among HIV+ adults, higher levels of the proinflammatory cytokine interleukin-6 (IL-6) are strongly associated with risk for CVD, cancer, progression to AIDS, and death (Borges et al., 2016), and IL-6 and C-reactive protein (CRP) are strongly and independently associated with all-cause mortality (Kuller et al., 2008).
Stress is a well-established contributor to inflammation and immune dysregulation in healthy, non-HIV infected adults (Gouin, Hantsoo, & Kiecolt-Glaser, 2008). Though preclinical studies show a consistent increase in glucocorticoids and inflammatory markers in response to stress and provide a model of appropriately functioning glucocorticoid-immune dynamic where the former dampens the latter (Kunz-Ebrecht, Mohamed-Ali, Feldman, Kirschbaum, & Steptoe, 2003), the relationship in humans varies considerably (Bale & Epperson, 2015). Age, reproductive status, previous exposures to adversity and trauma, type of laboratory stressor, as well as health and psychological status at the time of assessment are just a few of the many factors that contribute to variations in physiologic responsiveness to acute laboratory, and presumably naturally occurring, stressors in humans (Steptoe, Hamer, & Chida, 2007). Individuals, particularly women, living with HIV are more likely to have experienced traumatic life events than HIV- individuals (Machtinger, Wilson, Haberer, & Weiss, 2012), a factor that is associated with a plethora of negative health outcomes (Felitti et al., 1998) and altered glucocorticoid-immune status (do Prado, Grassi-Oliveira, Daruy-Filho, Wieck, & Bauer, 2017). Surprisingly, few studies have assessed the impact of stress on markers of inflammation or glucocorticoid regulation of inflammation in individuals living with HIV, particularly women. Emphasizing the potential importance to long-term health, HIV+ men classified as having relatively high cortisol levels and experiencing severe life stress in the preceding six months had lower counts of CD8+, CD16+, CD56+ and CD57+ cells than those experiencing severe stress in the lower cortisol group (Petitto et al., 2000). HIV+ men had an elevated cortisol and blunted immune response to an acute laboratory stressor compared with HIV- men (Hengge, Reimann, Schäfer, & Goos, 2003), although HIV+ women were not included. This is notable given that mid-life women experience an HIV disease course characterized by accelerated age-related increases in inflammatory chemokines relative to men (Martin et al., 2013) and vulnerability to SNAEs such as HAND, which is more prevalent in HIV+ women than HIV+ men (Maki & Martin-Thormeyer, 2009). There is some evidence that women living with HIV progress to menopause, a state of greater inflammation and accentuated cortisol response to stress (Bale & Epperson, 2015; Kajantie & Phillips, 2006) at a more rapid rate than HIV- women suggesting that they would be particularly vulnerable to the adverse effects of stress on health outcomes including cognitive function (Rubin et al., 2014; Schoenbaum et al., 2005).
The current research was undertaken within a larger study examining cognitive differences by HIV serostatus in mid-life HIV+ and HIV- individuals. Using this convenience sample of mid-life individuals with and without HIV, we aimed to investigate sex differences by serostaus in cytokines (IL-6, IL-8, IL-10, IL-1β, CRP, tumor necrosis factor (TNF)-α, interferon (IFN)-γ)) and cortisol in response to an acute laboratory stressor. The stressor, Grillon’s no shock, predictable, unpredictable (NPU) threat of shock task (Schmitz & Grillon, 2012), was chosen as threat paradigms have mixed effects on physiologic markers of stress (e.g. salivary cortisol, heart rate, skin conductance), suggesting a more variable response in humans (Grillon et al., 2011; Miller, McKinney, Kanter, Korte, & Lovallo, 2011). We hypothesized that mid-life HIV+ women would exhibit dysregulated glucocorticoid-immune response to acute stress.
METHODS
PARTICIPANTS
Participants aged 40–55 years were recruited from the community. Inclusion criteria required HIV+ individuals to have been living with HIV for at least the last three years, and HIV- individuals to report negative serostatus and no exposure risk factor for the past ten years. Exclusion criteria included pregnancy, steroid medications or beta-blockers, recreational drug use, current major depression or anxiety disorder, lifetime history of psychotic or bipolar disorder, drug dependence or abuse within the past 5 years, history of seizures, dementia or mild cognitive impairment.
STUDY PROTOCOL
Individuals completed a brief telephone interview to assess eligibility criteria. Eligible individuals presented at the laboratory for Visit 1 in which they provided written informed consent, provided a brief medical history, and completed the self-report questionnaires listed below. Pre-menopausal female participants completed a urine pregnancy test (Sure-Vue Serum/Urine hCG Test; Fischer Scientific, Pittsburgh, PA), and all participants completed a urine drug screen (iCup Urine Test; Alere Instant Technologies; Waltham, MA). Participants returned for Visit 2, in which the laboratory stressor was completed. An intravenous line (IV) was placed to allow serial blood collection. Participants rested quietly for 30 minutes, then completed the laboratory stressor; a threat of shock procedure in which mild shocks were applied during a 45-minute task. Blood was sampled at time points listed below for measurement of serum cortisol and inflammatory markers. Participants were reimbursed $50 for study screening and $100 for study completion. Study procedures were approved by the University of Pennsylvania School of Medicine Institutional Review Board (IRB) and performed in accordance with the Helsinki Declaration of 1975.
Study Instruments.
Demographic information was collected including age, education, marital status, race, ethnicity, income, and insurance status. Patients provided information about family history of psychiatric disorders, alcoholism, and nicotine dependence. The Structured Clinical Interview for DSM-IV (SCID) was administered by trained research staff to assess lifetime and current psychiatric disorders. The Hamilton Depression Scale (HAM-D) was administered to assess depressive symptoms. Participants completed the Beck Depression Inventory (BDI-II), Short Form Health Survey (SF-12), Perceived Stress Scale (PSS), and State-Trait Anxiety Scale - State (STAI-S) to assess anxiety at baseline and in response to the stress task.
Threat of Shock Laboratory Stressor.
Grillon’s no shock, predictable, unpredictable (NPU) threat of shock task was utilized as a laboratory stressor (Schmitz & Grillon, 2012). The stressor task occurred between 12:00 and 2:00 p.m. Participants were asked to abstain from benzodiazepines for 48 hours and nicotine for one hour prior to the task, confirmed with a breath carbon monoxide measurement. Participants were provided a description of the task, which included a series of visual cues indicating the likelihood of receiving a mild shock. A series of eight sample shocks without cues was administered in a graded manner (1–5 mA) to determine a level of shock that was unpleasant but not painful for that participant. A habituation trial of four startle probes delivered every 18–25 seconds was completed to reduce excessive initial startle reactivity. Acoustic startle probes were 50ms white noise bursts at 103dB with zero rise time presented through circumaural earphones (EARTone Auditory Systems, 3M, St. Paul, MN). Mild brief shocks (100 milliseconds) were produced by a constant current stimulator applied to the wrist (Psychlab, Contact Precision Instruments, London, UK). A total of twelve shocks were administered over approximately 45 minutes. Participants had a 5 minute break mid-task, at which time s/he completed the STAI-S. After the NPU task was completed, participants completed the STAI-S again.
Blood Samples.
Blood was collected from the IV line at −30, −15, +20, +45, +75 and +105 minutes (T=0 as start time of the laboratory stressor) to assess cortisol and cytokines; viral load copies were also measured.
Cytokine and Cortisol Assays.
Assessment of high sensitivity (hs)IL-1β, hsIL-6, hsTNF-α, hsIL-8, hsIL-10, and hsIFN-γ in serum was performed using a solid phase protein immunoassay with spectrally encoded antibody-conjugated beads as the solid support (Luminex). CRP and cortisol were assessed in serum samples using a solid phase sandwich enzyme linked-immuno-sorbent assay (ELISA).
Statistical Analyses.
Data were inspected to evaluate normality and other model assumptions. Independent sample T-tests were completed to determine differences in demographics, self-report measures, and baseline hormone and immune factors between HIV- and HIV+ groups. A linear mixed model extension of the repeated measures ANOVA model (Zeger & Liang, 1986) was used to examine impact of sex, HIV status and time on cytokines and cortisol levels over six timepoints from pre- to post-stressor. Cytokine and cortisol values were log transformed to better approximate a normal distribution.
RESULTS
Sample Characteristics.
Participants included 8 HIV- controls (n=5 female) and 9 HIV+ individuals (n=5 female). The groups were similar in demographic characteristics such as age, race, and education (Table 1). Three of the women were pre-menopausal (distributed across HIV groups), and seven were postmenopausal. All HIV+ individuals were virally suppressed based on <20 copies/mL viral load.
Table 1.
Demographic and health characteristics of the sample.
| HIV- | HIV+ | p-value | |
|---|---|---|---|
| Sex (n, %) | 0.77 | ||
| Male | 3 (37.5) | 4 (44.4) | |
| Female | 5 (62.5) | 5 (55.6) | |
| Age (Mean, S.D.) | 50.1 (2.6) | 48.9 (4.2) | 0.44 |
| Race (n, %) | 0.23 | ||
| Caucasian | 4 (50) | 2 (22.2) | |
| African American | 4 (50) | 7 (77.8) | |
| Education (n, %) | 0.28 | ||
| High school or less | 2 (28.6) | 5 (55.6) | |
| Some college | 5 (71.4) | 4 (44.4) | |
| Copies Viral Load <20/mL (n, %) | N/A | 9 (100) | N/A |
| BDI (Mean, S.D.) | 5.5 (6.3) | 1.8 (1.9) | 0.15 |
| PSS | 12.0 (3.54) | 12.33 (5.64) | 0.89 |
| STAI-S Pre-Stressor | 25.88 (4.61) | 31.78 (11.36) | 0.19 |
| STAI-S Mid-Stressor | 36.5 (7.4) | 33.0 (9.7) | 0.42 |
| STAI-S Post-Stressor | 32.4 (6.9) | 33.8 (10.7) | 0.76 |
Self-report Psychological Measures.
HIV- and HIV+ participants were similar in all self-report psychological measures (Table 1). The STAI-S increased from pre- to mid-stressor and post-stressor in both groups, verifying that the NPU task was subjectively stressful (p=0.03).
Cortisol Response.
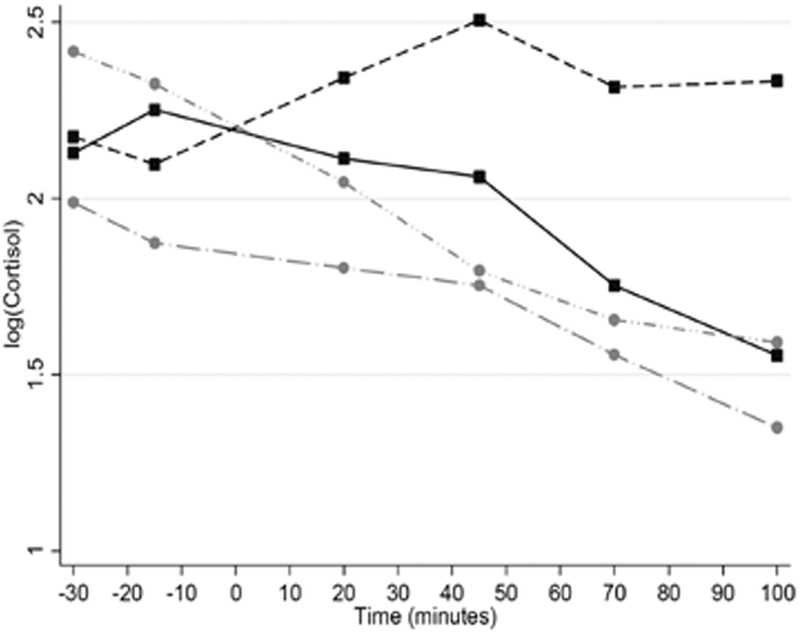
A sex x HIV status x time interaction indicated that cortisol increased over time among HIV+ men compared to HIV- men and all women (χ2 (5) = 11.85, p=0.037) (Figure 1).
Figure 1. Cortisol response to stress.
A sex by HIV status by time interaction indicated that in response to a laboratory stressor, cortisol increased over time among HIV+ men compared to HIV- men and all women (p=0.037).
Cytokine Response.
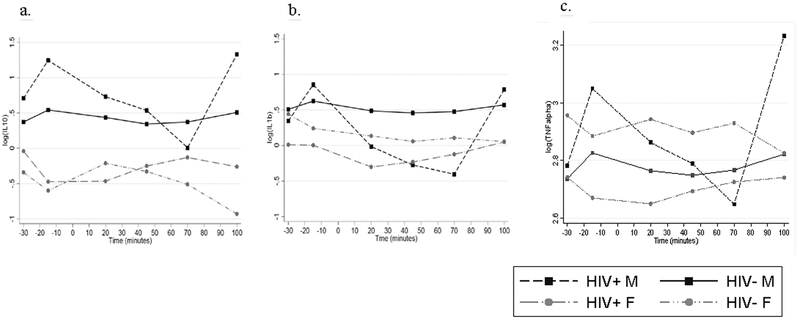
Repeated measures mixed models revealed a significant sex x HIV status x time interaction for IL-10 (χ2 (5) = 16.76, p=0.005), IL-1ß (χ2 (5) = 22.67, p=0.0004), and TNF-α (χ2 (5) = 24.45, p=0.0002) (Figure 2). There were no significant three-way interactions for IL-6 (χ2 (5) = 7.97, p=0.158), CRP (χ2 (5) = 5.29, p=0.382), IFN- γ (χ2 (5) = 4.72, p=0.451), IL-8 (χ2 (5) = 8.72, p=0.121). Levels did not differ by HIV status for IL-6 (X2(1)=−0,162, p=0.477), CRP (X2(1)=0,552, p=0.446), IFN-γ (X2(1)=−0,252, p=0.618) or IL-8(X2(1)=−0,180, p=0.427).
Figure 2. Inflammatory response to stress.
a) IL-10 response was larger in males than females, regardless of HIV status (p=0.005). b) HIV+ individuals, particularly women, had a blunted IL-1ß response to stress compared with HIV- controls (p=0.0004). c) TNF-α response was smaller in HIV+ individuals than HIV-, regardless of sex (p=0.0002).
DISCUSSION
The principal novel finding in this sample of mid-life adults, is the impact of sex and HIV status on glucocorticoid and immune response to a threat-based laboratory stressor such that HIV+ males were the only group to mount a cortisol and cytokine response despite all groups self-reporting similar and expected levels of psychological distress across a threat-based stress paradigm. We had expected the sex x HIV status interaction to be driven by the HIV+ female group given their age, predominantly postmenopausal status and the typical HIV-associated pro-inflammatory state. Instead, cortisol response among the HIV+ women could not be distinguished from the rather flat cortisol profile of the of HIV- males and females suggesting that the NPU paradigm was sufficient to trigger psychological distress, but no appreciable change in cortisol secretion. Our cortisol findings in HIV+ males are similar to those of Hengge et al (2003), in which HIV+ men showed a larger cortisol response to acute stress. While both control groups in our study showed no appreciable cortisol response, a flat stress response among HIV+ women may be unfavorable given the relatively higher inflammatory profile that is common among individuals living with HIV. This theory is supported by research showing that lower nocturnal urinary cortisol in HIV+ compared to HIV- women is linked with increased risk for coronary heart disease (Dale, Weber, Cohen, & Brody, 2017).
With respect to cytokine profiles, the rather robust sex x HIV status change in cytokine response to stress was not in the anticipated direction as HIV+ individuals, particularly women, had a fairly flat IL-1ß response to stress compared with HIV- controls. Typically, IL-1ß increases in response to acute stress, but among these HIV+ women it did not, despite subjective increases in anxiety. IL-10 response was larger in males than females, regardless of HIV status. IL-10 is an anti-inflammatory cytokine, and a dampened IL-10 response to acute stress among women may thus be unfavorable. Again, unexpectedly, TNF-α response was smaller in HIV+ individuals than HIV-. Elevated TNF-α has been associated with HIV somatic symptoms and detectable viral load (Norcini Pala et al., 2016), not characteristic of this relatively healthy HIV+ sample.
This study has the obvious limitation of a small sample size making consideration of factors such as current gonadal hormone levels and previous history of childhood adversity or lifetime trauma exposures, both factors that impact glucocorticoid and immune response to stress (Morrison et al., 2017) impractical. In addition, female participants included pre and postmenopausal women, and menstrual cycle phase was not assessed for the three premenopausal participants. Given the majority of women were postmenopausal, a state that is associated with heightened cortisol response to stress (Bale & Epperson, 2015; Kajantie & Phillips, 2006) and the 3 premenopausal women were across HIV groups and unlikely to all be in a high estradiol state, our finding of a decline in cortisol in females across the stress paradigm is unlikely to be impacted by the mixed reproductive status of the sample. Future research should also consider testosterone levels in HIV+/HIV- males as testosterone dampens stress responsiveness and levels can be reduced in HIV+ males (Wong, Levy, & Stephenson, 2017). All HIV+ individuals were virally suppressed, indicating that their disease was well controlled; viral load has been found to be a moderator between psychological distress and immune function in HIV+ individuals (Motivala et al., 2003). Finally, future studies may benefit from utilizing a social evaluative stressor as they more reliably evoke a robust cortisol response, though in our studies individuals reporting substantial childhood adversity show a blunted response to a socially relevant stressor (Morrison et al., 2017). The study’s strengths included a well-matched control group with both men and women represented, and thorough psychological assessments to ensure that participants were psychiatrically healthy. In sum, our results underscore the importance of including women in HIV research, as sex differences in immune parameters have implications for outcomes such as CVD and HAND in HIV+ women (Valdez, Rubin, & Neigh, 2016).
Acknowledgements.
We acknowledge Richard Tustin III, Nancy Tustin and Laura A. Schankel for performing cytokine and cortisol assays.
Funding: This study was funded by The University of Pennsylvania Perelman School of Medicine’s Mental Health AIDS Research Center, National Institute of Mental Health (NIMH) P30 MH097488 and K24 DA030301 (Epperson).
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- Bale TL, & Epperson CN (2015). Sex differences and stress across the lifespan. Nature Neuroscience, 18(10), 1413–1420. 10.1038/nn.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges ÁH, O’Connor JL, Phillips AN, Neaton JD, Grund B, Neuhaus J, … INSIGHT SMART Study and ESPRIT Groups. (2016). Interleukin 6 Is a Stronger Predictor of Clinical Events Than High-Sensitivity C-Reactive Protein or D-Dimer During HIV Infection. The Journal of Infectious Diseases, 214(3), 408–416. 10.1093/infdis/jiw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale SK, Weber KM, Cohen MH, & Brody LR (2017). Abuse, nocturnal stress hormones, and coronary heart disease risk among women with HIV. AIDS Care, 29(5), 598–602. 10.1080/09540121.2016.1241378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Prado CH, Grassi-Oliveira R, Daruy-Filho L, Wieck A, & Bauer ME (2017). Evidence for Immune Activation and Resistance to Glucocorticoids Following Childhood Maltreatment in Adolescents Without Psychopathology. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(11), 2272–2282. 10.1038/npp.2017.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Gouin J-P, Hantsoo L, & Kiecolt-Glaser JK (2008). Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation, 15(4–6), 251–259. 10.1159/000156468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Heller R, Hirschhorn E, Kling MA, Pine DS, Schulkin J, & Vythilingam M (2011). Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study. Biological Psychiatry, 69(6), 549–555. 10.1016/j.biopsych.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge UR, Reimann G, Schäfer A, & Goos M (2003). HIV-positive men differ in immunologic but not catecholamine response to an acute psychological stressor. Psychoneuroendocrinology, 28(5), 643–656. [DOI] [PubMed] [Google Scholar]
- Hong S, & Banks WA (2015). Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain, Behavior, and Immunity, 45, 1–12. 10.1016/j.bbi.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, & Phillips DIW (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31(2), 151–178. 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, … INSIGHT SMART Study Group. (2008). Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Medicine, 5(10), e203 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, & Steptoe A (2003). Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain, Behavior, and Immunity, 17(5), 373–383. [DOI] [PubMed] [Google Scholar]
- Machtinger EL, Wilson TC, Haberer JE, & Weiss DS (2012). Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS and Behavior, 16(8), 2091–2100. 10.1007/s10461-011-0127-4 [DOI] [PubMed] [Google Scholar]
- Maki PM, & Martin-Thormeyer E (2009). HIV, cognition and women. Neuropsychology Review, 19(2), 204–214. 10.1007/s11065-009-9093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, … Crowe SM (2013). Age-Associated Changes in Monocyte and Innate Immune Activation Markers Occur More Rapidly in HIV Infected Women. PLoS ONE, 8(1). 10.1371/journal.pone.0055279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, McKinney AE, Kanter FS, Korte KJ, & Lovallo WR (2011). Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology, 36(7), 970–980. 10.1016/j.psyneuen.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, … Bale TL (2017). Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biological Psychiatry, 81(8), 693–701. 10.1016/j.biopsych.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Hurwitz BE, Llabre MM, Klimas NG, Fletcher MA, Antoni MH, … Schneiderman N (2003). Psychological distress is associated with decreased memory helper T-cell and B-cell counts in pre-AIDS HIV seropositive men and women but only in those with low viral load. Psychosomatic Medicine, 65(4), 627–635. [DOI] [PubMed] [Google Scholar]
- Norcini Pala A, Steca P, Bagrodia R, Helpman L, Colangeli V, Viale P, & Wainberg ML (2016). Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain, Behavior, and Immunity, 56, 105–113. 10.1016/j.bbi.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto JM, Leserman J, Perkins DO, Stern RA, Silva SG, Gettes D, … Evans DL (2000). High versus low basal cortisol secretion in asymptomatic, medication-free HIV-infected men: differential effects of severe life stress on parameters of immune status. Behavioral Medicine (Washington, D.C.), 25(4), 143–151. 10.1080/08964280009595743 [DOI] [PubMed] [Google Scholar]
- Rubin LH, Sundermann EE, Cook JA, Martin EM, Golub ET, Weber KM, … Maki PM (2014). Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause (New York, N.Y.), 21(9), 997–1006. 10.1097/GME.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, & Grillon C (2012). Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols, 7(3), 527–532. 10.1038/nprot.2012.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum EE, Hartel D, Lo Y, Howard AA, Floris-Moore M, Arnsten JH, & Santoro N (2005). HIV infection, drug use, and onset of natural menopause. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 41(10), 1517–1524. 10.1086/497270 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Valdez AN, Rubin LH, & Neigh GN (2016). Untangling the Gordian knot of HIV, stress, and cognitive impairment. Neurobiology of Stress, 4, 44–54. 10.1016/j.ynstr.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N, Levy M, & Stephenson I (2017). Hypogonadism in the HIV-Infected Man. Current Treatment Options in Infectious Diseases, 9(1), 104–116. 10.1007/s40506-017-0110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, & Liang KY (1986). Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 42(1), 121–130. [PubMed] [Google Scholar]