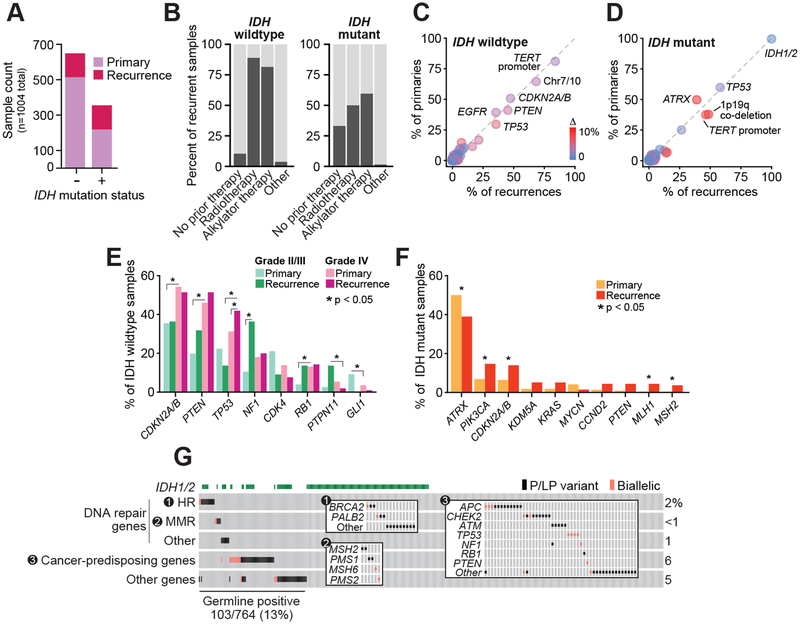
Figure 1. Clinical and genomic characteristics of prospectively characterized gliomas.
A) The number of primary (n=733; 219 IDH-mutant and 514 IDH WT) and recurrent tumor specimens (n=271; 136 IDH-mutant and 135 IDH WT) characterized in the study cohort are shown by IDH-mutation status. B) The percentage of recurrent specimens acquired at any point after one or more lines of the indicated therapeutic modality (91 of 136 of IDH-mutant and 121 of 135 IDH WT recurrences acquired after at least one line of treatment). C-D) The frequency of key glioma-associated mutations in primary and recurrent IDH-WT or IDH-mutant tumors indicate glioma subtype-defining lesions are largely stable over time and therapy. Cardinal genomic lesions are labeled, circles are colored by absolute difference in frequency between primary and recurrent tumors (IDH wildtype: n=514 and 135 primary and recurrent tumors, respectively; IDH-mutant: n=219 and 136 primary and recurrent tumors, respectively). E-F) The frequency of aberrations in those genes with differing frequencies (P<.05 or a 2.5 fold-change) in IDH WT and IDH-mutant tumors (panels E and F, respectively) across grade or sample type (primary versus recurrence). IDH WT: 80, 420 and 14 primary tumors of grades II/III, IV or mixed/indeterminate grade, respectively, and 12, 106, and 17 recurrent tumors of grades II/III, IV or mixed/indeterminate grade; IDH-mutant: 218 and 136 primary and recurrent tumors, respectively). G) The frequency and pattern of pathogenic and likely pathogenic abnormalities in the germline of patients with IDH-WT or -mutant disease is shown. Inset panels highlight specific subgroups genes by pathway of interest. Somatic biallelic loss following a germline lesion is indicated by pink squares. DNA repair genes labeled as “Other” are ATR, ERCC2, ERCC5 and MUTYH.