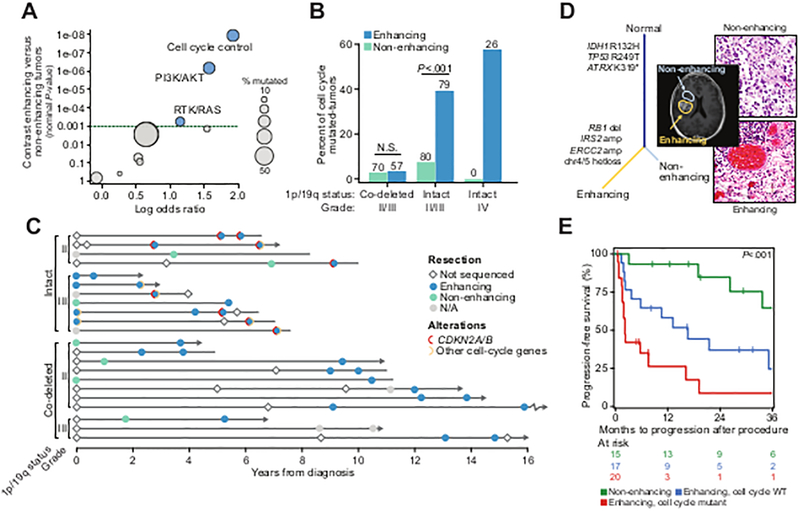
Figure 2. Genomic correlates of contrast-enhancement on brain imaging in IDH-mutant tumors.
A) Genomic alterations in core molecular and signaling pathways are associated with enhancing disease in IDH-mutant gliomas [n=150 and 148 non-enhancing and enhancing primary and recurrent tumors (111 and 39 versus 77 and 71, respectively) and recurrent tumors, respectively; n=42 and 270 sequenced grade IV and grade II/III specimens, respectively). All but 24 tumors were grade II-III at diagnosis. B) The rate of alterations in effectors of the cell cycle across IDH-mutant glioma of different grade and 1p19q status (N cases indicated above each bar), and by presence of enhancement on presurgical MRI. P-values indicate significance, Fisher’s exact test. C) The molecular and clinical evolution in 22 patients with IDH-mutant disease [n=11 each of 1p19q-intact (four and seven grade II and III) and co-deleted (eight and three grade II and III), respectively] in the cohort with multiple specimens sequenced over time, grouped by grade and 1p19q status, indicates the temporal association of enhancing disease and the acquisition of a lesion in a cell-cycle effector. Arrows indicate patients still alive at last follow-up, lines without arrows indicate deceased patients. D) In a representative de novo IDH-mutant secondary GBM, two spatially distinct components of the primary tumor were profiled, one non-enhancing lower grade focus and one grade IV enhancing lesion. While clonally related (as represented by sample tree showing truncal mutations in IDH1, TP53 and ATRX), only the enhancing component possessed the cell cycle lesion (RB1 homozygous deletion). E) In IDH-mutant 1p19q intact tumors (n=47 and 18 grade II and III at diagnosis), progression-free survival from the time of recurrent surgery is significantly shorter in patients whose recurrence exhibit pre-surgical MRI enhancement and whose tumors harbored a cell-cycle alteration. Multivariate statistics from Cox proportional hazards model are presented in Fig. S6B.