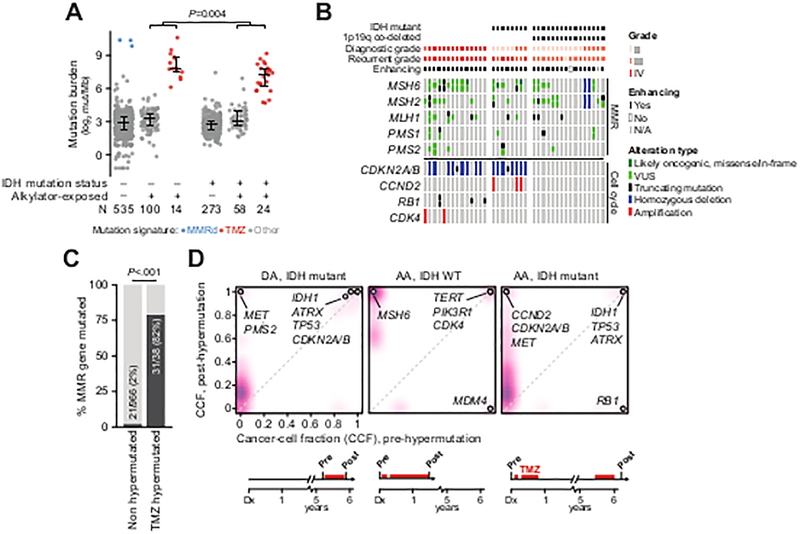
Figure 3. Genomic context of somatic hypermutation in glioma.
A) Somatic mutation rates in IDH-wildtype and IDH-mutant samples acquired prior to or after exposure to alkylator-based chemotherapy. Samples with elevated mutation burden were attributable to either mismatch repair (MMR) deficiency or prior alkylator exposure as reflected in their mutational signatures. B) TMZ-hypermutated gliomas (n=14 IDH WT, 8 IDH-mutant 1p19q-intact, and 16 IDH-mutant 1p19q co-deleted; grade at diagnosis and recurrence as indicated) have nearly obligate lesions in effectors of MMR independent of the status of IDH, 1p19q, or diagnostic and recurrent grade, whereas cell-cycle lesions (predominantly copy-number alterations and not mutations) arose only in 1p19q intact tumors independent of IDH status and diagnostic grade. C) Mutations in MMR genes occur infrequently in gliomas that do not develop alkylator-mediated hypermutation as compared to those with therapy-induced hypermutation. D) In three representative patients with matched pre-treatment and post-TMZ specimens, clonal evolution of a TMZ-hypermutated clone was evident (purple is proportional to the density of somatic mutations arising at a given cancer cell fraction). In all cases, a cell-cycle abnormality precedes therapy (labeled). The abbreviated clinical course for each patient is shown at bottom. DA: diffuse astrocytoma, AA: anaplastic astrocytoma.