Abstract
There is extensive debate regarding the protective effect of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) on colorectal cancer (CRC). We aimed to assess the association between CRC risk and exposure to statins using a large cohort with prescription data. We carried out a case-control study in Catalonia using the System for Development of Primary Care Research (SIDIAP) database that recorded patient diseases history and linked data on reimbursed medication. The study included 25 811 cases with an incident diagnosis of CRC between 2010 and 2015 and 129 117 frequency-matched controls. Subjects were classified as exposed to statins if they had ever been dispensed statins. Analysis considering mean daily defined dose, cumulative duration and type of statin were performed. Overall, 66 372 subjects (43%) were exposed to statins. There was no significant decrease of CRC risk associated to any statin exposure (OR = 0.98; 95% CI: 0.95–1.01). Only in the stratified analysis by location a reduction of risk for rectal cancer was observed associated to statin exposure (OR = 0.87; 95% CI: 0.81–0.92). This study does not support an overall protective effect of statins in CRC, but a protective association with rectal cancer merits further research.
Subject terms: Disease prevention, Epidemiology, Colorectal cancer, Risk factors
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide1 and its incidence is still rising in many low and middle income countries2. Focus on primary prevention and screening is necessary in order to reduce the incidence and mortality of this cancer. Although lifestyle risk factors have been identified in CRC3, randomized trials have failed to show a reduction of adenomas recurrence with diet4–6 or dietary supplements7,8. A large body of evidence has shown that nonsteroidal anti-inflammatory drugs (NSAID), particularly acetylsalicylic acid (ASA), reduce the risk of colorectal neoplasia9,10 but with possible adverse events11. Indeed, a safe and effective CRC chemoprevention agent in average-risk population would help reducing the incidence of colorectal neoplasia.
Statins, inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, are a widely used and well-tolerated class of drugs for the treatment of hypercholesterolemia. Previous studies indicate their possible role in cancer chemoprevention12,13, with controversial results14–16. In addition to their main effect on cholesterol synthesis, statins may cause a number of other pleiotropic effects that may influence tumorigenesis, such as antioxidant activity, effects on cell adhesion, or angiogenesis17. In vitro, statins have shown anti-proliferative and pro-apoptotic effects on human CRC cell lines, and also in tumour xenograft models18,19. Studies analysing the effects of exposure to statins on the prevention or prognosis of colorectal neoplasia have shown controversial results, which have been proposed to be due to heterogeneity amongst drugs, or to effects restricted to some subgroup of patients14–16,20.
In this observational study we have analysed a population-based health records database aiming to examine the association between statins, their subtypes and pattern of use, and CRC risk.
Methods
Data source
Subjects were selected from the Information System for Development of Primary Care Research (SIDIAP) database (www.sidiap.org)21, which comprises clinical information routinely collected by primary care professionals of the Catalan Institute of Health. This database includes information from 5.8 million people in Catalonia (almost 80% of the population) that have ever contacted the public health system since 2005. The data retrieved included routine clinical data, such as diagnoses and health measurements, and was linked to information on dispensed prescriptions generated by pharmacies’ claims for reimbursement by the Catalan Health System. Drugs were coded according to the Anatomical Therapeutic Chemical (ATC) classification system22, and the date and quantity of the drug withdrawn from the pharmacy were recorded. Irreversible encoding of patient identifiers ensured anonymization of the information in the SIDIAP study database. The quality of SIDIAP data has been previously documented, and it has been used to study the epidemiology of health outcomes23.
All procedures performed in the study involving data from human participants were in accordance with the ethical standards of the institutional research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. No informed consent was requested to the participating individuals, since this study was based on anonymized data routinely collected. No variables with potential to identify specific individuals were retrieved. The study protocol was approved by the Ethics Committee for Clinical Research of IDIAP Jordi Gol and all applicable regulatory requirements were fulfilled. The study was registered in ENCePP database with code EUPAS12697.
Study design
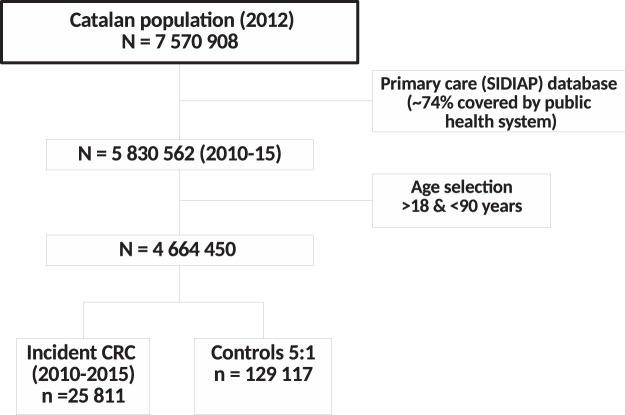
A population-based case-control study nested within the cohort of subjects receiving primary care from the Institut Català de la Salut was conducted. The flow chart of the study is described in Fig. 1. The cohort of subjects registered in SIDIAP with at least one healthcare interaction in last 3 years (n = 5 830 562) was limited to adult population, aged 18 to 90 years (n = 4 664 450). Cases identified with a recorded incident diagnosis of colon or rectum (codes C18, C19, and C20 of the International Classification of Diseases 10th Revision [ICD-10]) within the period January 1, 2010 to December 31, 2015 were identified. Those cases with a diagnosis of appendix cancer (C18.1) were excluded to avoid the inclusion of carcinoid tumours which are more frequent in that location.
Figure 1.
Population flowchart and study design. SIDIAP includes subjects that have interacted with the Catalan public health system (~74% of the total Catalan population). The study selected all CRC patients in the period 2010–2015 aged 18 to 90 years. Cases were incident diagnosis of colon or rectum (ICD-10 codes C18, C19, and C20). For each case, 5 matched controls of the same sex, age ± 5 years and health area were selected and assigned the case diagnosis date as index date for exposure assessment. Appendix cancer (C18.1) cases were excluded.
A random stratified selection of controls was obtained using the same SIDIAP database. For each case, five controls were randomly selected from the set of all subjects in the database without prior CRC and alive at the time of diagnosis of the case, with the same age (±5 years) and sex and living in the same region defined by the primary healthcare centre catchment area. For cases, the disease onset date, defined as the earliest CRC diagnosis date registered, was set as the index date. For controls, the index date of their matched case was applied. Information regarding comorbidities and drug use was truncated to that recorded prior to the index date for cases and controls.
To assess if the codes used for case identification were reliable and exhaustive, and also to obtain an indirect measure of the external validity of our sample, we estimated the expected number of incident CRC cases in the population covered by the database according to cancer registries in Catalonia that contribute to the International Agency for Research on Cancer (IARC) publication Cancer Incidence in Five Continents (CI5) Volume XI24. The age-specific incidence rates of colon and rectum cancer estimated in the Tarragona and Girona cancer registries for 2012 were downloaded from https://ci5.iarc.fr. The age-specific rates were averaged over registries and summed for colon and rectum, then multiplied by the total Catalan population for the same age groups and period downloaded from https://www.idescat.cat. The population was corrected multiplying by 0.8 to account the average SIDIAP cumulative coverage on the studied period. Supplementary Fig. 1 shows that the number and age-sex distribution of the cases observed was similar to those expected.
Exposure variables
Patients were classified as exposed to statins if they had retrieved at least one dispensation with an Anatomical Therapeutic Chemical code beginning with “C10AA”; otherwise, they were classified as unexposed. We also obtained exposure data for nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin (M01A, N02BA, N02BB, and B01AC06) as they potentially could confound the association between statin use and cancer risk, and other lipid lowering drugs (C10AB, C10AC, C10AD, C10AX). Daily defined doses (DDD) for each dispensed prescription were calculated by multiplying the container pills by dose (in mg) and dividing by the World Health Organization defined DDD (in mg) for each individual drug22. The average dose for each of these duration categories was established, dividing the sum of DDD by the interval length. Finally, to measure the effect of timing of exposure, we compared non-users to the subjects exposed exclusively 1 month to 5 years before the index date (short exposure) and the subjects exposed throughout the period of more than 5 years before the index date (long exposure). We chose ≥5 years as a cut-off point of long term, according to the mean of follow-up and statin use in randomized controlled trials.
In order to compare the results with previous studies, statins were classified as lipophilic (atorvastatin, fluvastatin, lovastatin, simvastatin) or hydrophilic (pravastatin and rosuvastatin) and, by effectiveness in lowering LDL cholesterol levels, as low-potency (fluvastatin, lovastatin, pravastatin, simvastatin) and high-potency (atorvastatin and rosuvastatin)25.
Confounders
The potential confounders identified a priori for this analysis were age, sex, socioeconomic status, region, year, body mass index (BMI), tobacco, alcohol, comorbidity conditions and NSAID use3,9,10. Socioeconomic status was evaluated using the MEDEA socioeconomic deprivation score26, which was divided into quintiles for the analysis. Chronic comorbidity conditions considered for multivariable adjustment included those associated with CRC in the data: hypertension, hyperuricemia, diabetes, osteoarthritis and spondyloarthropathy, chronic lower respiratory diseases, extrapyramidal and movement disorders, episodic and paroxysmal disorders, mental and behavioural disorders, chronic kidney disease, heart failure, cerebrovascular disease, liver disease, insomnia, osteoporosis, peptic ulcer, inflammatory bowel disease. Conditions clearly defined as indications of statins (dyslipidaemia and cardiovascular disease) were not considered for adjustment, since they are in the explored causal path.
Statistical analysis
Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated from unconditional logistic regression models. We compared the effect of no use to any use of drug and assessed effects of dose (DDD) and duration of statin use. We also explored the effect of the type of the statin (potency and lipophilicity). Subgroup analyses were performed according to sex, age groups, NSAID use and cancer location (colon or rectum). Missing data for body mass index (BMI) was imputed using the prediction of a linear model according to age, sex and, outcome status (63% had complete data). To avoid models with many parameters, an adjustment score was built from the predictions of a logistic regression model for CRC that included all potential confounding covariates, without selection strategies. This adjustment score was efficient to render all potential confounders non-significantly associated to CRC. All logistic regression models included the adjustment score. P-values were derived from likelihood ratio tests. Statistical analysis was carried out using R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and statin exposure
Characteristics of the study population according to statin use are presented in Table 1. During the study period there were 25 811 CRC cases which were matched by sex, age at time of index date (±5 years), and healthcare region to 129 117 controls. A total of 66 372 (42.8%) subjects were ever users of 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors at the index date, 55 008 (42.6%) were controls and 11 364 (44.0%) CRC cases. Statin users were older with only 3.2% being less than age 55 years at entry (median age 74 years for users and 67 years for non-users). Statin consumption was associated with age, male sex, data of entry in the cohort, higher BMI, former smoking, severe alcohol consumption and, higher NSAID prescription (Table 1). Statin users were more likely to have comorbidities (see Supplementary Table 1).
Table 1.
Characteristics of the study according to statin use (controls only, N = 129117).
| Characteristic | Statin non-users | Statin users | ORa | 95% CI | P-value (trend) | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Age | |||||||
| 18–55 years | 13076 | 17.6 | 1778 | 3.2 | 1 | ||
| 55–65 years | 17948 | 24.2 | 8793 | 16.0 | 3.59 | 3.40–3.80 | |
| 65–75 years | 17945 | 24.2 | 17560 | 31.9 | 7.17 | 6.79–7.56 | |
| 75–85 years | 18065 | 24.4 | 20912 | 38.0 | 8.50 | 8.06–8.97 | |
| 85–95 years | 7075 | 9.5 | 5965 | 10.8 | 6.23 | 5.86–6.62 | <0.0001 |
| Sex | |||||||
| Male | 43557 | 58.8 | 33112 | 60.2 | 1 | ||
| Female | 30552 | 41.2 | 21896 | 39.8 | 0.93 | 0.91–0.96 | <0.0001 |
| Year of entry (years) | |||||||
| 2010 | 12871 | 17.4 | 7743 | 14.1 | 1 | ||
| 2011 | 12913 | 17.4 | 8426 | 15.3 | 1.11 | 1.07–1.16 | |
| 2012 | 12884 | 17.4 | 9582 | 17.4 | 1.24 | 1.19–1.29 | |
| 2013 | 12754 | 17.2 | 10297 | 18.7 | 1.33 | 1.28–1.38 | |
| 2014 | 12858 | 17.4 | 10500 | 19.1 | 1.38 | 1.33–1.44 | |
| 2015 | 9829 | 13.3 | 8460 | 15.4 | 1.43 | 1.37–1.49 | <0.0001 |
| Body Mass Index | |||||||
| ≤25 kg/m2 | 12217 | 16.5 | 8769 | 15.9 | 1 | ||
| 25.1–30.0 kg/m2 | 47902 | 64.6 | 29691 | 54.0 | 0.91 | 0.88–0.94 | |
| >30 kg/m2 | 13990 | 18.9 | 16548 | 30.1 | 1.67 | 1.61–1.73 | <0.0001 |
| Tobacco b | |||||||
| Non-smoker | 35247 | 61.3 | 31761 | 61.0 | 1 | ||
| Current smoker | 10875 | 18.9 | 6608 | 12.7 | 0.94 | 0.91–0.98 | |
| Former smoker | 11392 | 19.8 | 13701 | 26.3 | 1.42 | 1.38–1.47 | |
| Alcohol b | |||||||
| None/mild | 30591 | 63.1 | 30927 | 64.5 | 1 | ||
| Moderate | 16524 | 34.1 | 16010 | 33.4 | 1.02 | 0.99–1.05 | |
| Severe | 1387 | 2.9 | 1045 | 2.2 | 0.89 | 0.81–0.96 | 0.004 |
| NSAIDs | |||||||
| Non-users | 31529 | 42.5 | 8525 | 15.5 | 1 | ||
| Users | 42580 | 57.5 | 46483 | 84.5 | 3.61 | 3.51–3.71 | <0.0001 |
aAdjusted for age and sex.
bVariables with missing data.
NSAID: Nonsteroidal anti-inflammatory drugs
The most frequent statin used was simvastatin (n = 48 907, 31.6% of all subjects) followed by atorvastatin (n = 25 198, 16.3% of all subjects) (Table 2). Of the 66 372 subjects ever exposed to any statin, 32 559 (49.1.5%) were long term users of statins (≥5 years). There were 22 995 (34.7%) individuals that were ever exposed to more than one different statin. The most common multiple exposure was simvastatin and atorvastatin, followed by simvastatin and pravastatin (Table 2).
Table 2.
Statin use and CRC risk.
| Controls | CRC cases | ORa | 95% CI | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Overall effect | Non-user | 74109 | 57.4 | 14447 | 56.0 | 1 | 0.11 | |
| Statin user | 55008 | 42.6 | 11364 | 44.0 | 0.98 | 0.95–1.01 | ||
| Durationb | Non-user | 74109 | 57.4 | 14447 | 56.0 | 1 | 0.0002 | |
| <5 years | 22515 | 17.4 | 4568 | 17.7 | 1.11 | 1.04–1.18 | ||
| ≥5 years | 27002 | 20.9 | 5557 | 21.5 | 0.96 | 0.93–1.00 | ||
| Cumulative dose | Non-user | 74109 | 57.4 | 14447 | 56.0 | 1 | 0.06 | |
| <709 DDD | 18344 | 14.2 | 3784 | 14.7 | 1.00 | 0.96–1.04 | ||
| 709–2370 DDD | 18307 | 14.2 | 3817 | 14.8 | 0.98 | 0.94–1.02 | ||
| >2370 DDD | 18357 | 14.2 | 3763 | 14.6 | 0.95 | 0.91–0.99 | ||
| Lipophilicity | Non-user | 74109 | 57.4 | 14447 | 56.0 | 1 | 0.38 | |
| Lipophilic | 45456 | 35.2 | 9418 | 36.5 | 0.98 | 0.95–1.01 | ||
| Hydrophilic | 2844 | 2.2 | 567 | 2.2 | 0.96 | 0.87–1.05 | ||
| Both | 6708 | 5.2 | 1379 | 5.3 | 0.96 | 0.91–1.03 | ||
| Statin potency | Non-user | 74109 | 57.4 | 14447 | 56.0 | 1 | 0.13 | |
| Low potency | 33139 | 25.7 | 6821 | 26.4 | 0.98 | 0.94–1.01 | ||
| High potency | 7900 | 6.1 | 1678 | 6.5 | 1.01 | 0.96–1.07 | ||
| Both | 13969 | 10.8 | 2865 | 11.1 | 0.96 | 0.91–1.00 | ||
| Type of statin | Simvastatin | 40584 | 31.4 | 8323 | 32.2 | 0.97 | 0.94–0.99 | 0.02 |
| Atorvastatin | 20839 | 16.1 | 4359 | 16.9 | 0.99 | 0.96–1.03 | 0.75 | |
| Pravastatin | 7964 | 6.2 | 1635 | 6.3 | 0.98 | 0.93–1.04 | 0.48 | |
| Fluvastatin | 3447 | 2.7 | 698 | 2.7 | 0.98 | 0.90–1.06 | 0.59 | |
| Lovastatin | 3132 | 2.4 | 677 | 2.6 | 1.04 | 0.96–1.13 | 0.35 | |
| Rosuvastatin | 1915 | 1.5 | 372 | 1.4 | 0.92 | 0.82–1.03 | 0.16 | |
| Pitavastatin | 325 | 0.3 | 69 | 0.3 | 1.03 | 0.79–1.34 | 0.83 | |
| Simvastatin + atorvastatinc | 11353 | 8.8 | 2355 | 9.1 | 0.97 | 0.93–1.02 | 0.29 | |
| Simvastatin + pravastatinc | 3973 | 3.1 | 832 | 3.2 | 1.00 | 0.92–1.08 | 0.95 | |
aAdjusted for age, sex, socioeconomic status, region, year, body mass index, smoking, alcohol, comorbidities and nonsteroidal anti-inflammatory drugs use.
bVariables with missing data.
cNon-users or users of only one of the two drugs as reference category.
A detailed table with the characteristics of the study population for cases of CRC and controls is presented in Supplementary Table 2. The environmental variables associated with CRC were lower BMI, former smoking and alcohol consumption. The median number of months on statin use was 58 months for both cases and controls.
Statin use and colorectal cancer
There was no overall association of statin use with CRC risk (OR = 0.98; 95% CI: 0.95–1.01, P = 0.11) (Table 2). The analysis of duration of exposure showed a significant 11% increase of risk for exposures to statins shorter than 5 years, while the analysis of cumulated exposure as derived from sum of DDDs per subject was not significant. Moreover, the risk of CRC was similar in patients with current or former exposure, and also among those who stopped taking statins 6 months, 12 months and 36 months before the index date.
No differences were observed when statins were classified by their potency or lipophilicity. The analysis of specific statins did not show differential effects regarding CRC risk. Finally, as Supplementary Table 3 shows, there was no interaction according to age groups (P-value for interaction = 0.06) nor gender (P-value for interaction = 0.09). There was a significant interaction for NSAIDs exposure, so that exposure to was protective in NSAIDs users but increased risk in non-users (P-value for interaction <0.01).
Statin use and rectal cancer
We performed a stratified analysis to determine whether CRC location influenced the effect of statins (Table 3). A statistically significant interaction of tumour location was observed (P < 0.001), with a significant reduction of 13% in the risk of rectal cancer (adjusted OR = 0.87; 95% CI: 0.81–0.92, P < 0.001), but not of colon cancer (adjusted OR 1.00; 95% CI: 0.97–1.03, P = 0.91). However, no consistent dose-effect was seen when analysing duration and dose. All types of statins showed similar significant associations for rectal cancer.
Table 3.
Analyses of statins effect according to CRC location.
| Controls | Colon cases | P-valueb | Rectal cases | P-valueb | P-interactionc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ORa | 95% CI | n | % | ORa | 95% CI | |||||
| Overall effect | Non-user | 74109 | 57.4 | 11754 | 55.3 | 1 | 0.91 | 2693 | 58.9 | 1 | <0.001 | <0.001 | ||
| Statin user | 55008 | 42.6 | 9488 | 44.7 | 1.00 | 0.97–1.03 | 1876 | 41.1 | 0.87 | 0.81–0.92 | ||||
| Durationd | Non-user | 74109 | 57.4 | 11754 | 55.3 | 1 | 0.001 | 2693 | 58.9 | 1 | <0.001 | |||
| <5 years | 22515 | 17.4 | 3813 | 18.0 | 0.99 | 0.95–1.03 | 755 | 16.5 | 0.96 | 0.82–1.11 | ||||
| ≥5 years | 27002 | 20.9 | 4636 | 21.8 | 0.98 | 0.95–1.02 | 921 | 20.2 | 0.85 | 0.79–0.93 | ||||
| Cumulative dose | Non-user | 74109 | 57.4 | 11754 | 55.3 | 1 | 0.10 | 2693 | 58.9 | 1 | <0.001 | |||
| <709 DDD | 18344 | 14.2 | 3167 | 14.9 | 1.03 | 0.98–1.07 | 617 | 13.5 | 0.87 | 0.80–0.96 | <0.001 | |||
| 709–2370 DDD | 18307 | 14.2 | 3202 | 15.1 | 1.01 | 0.97–1.06 | 615 | 13.5 | 0.85 | 0.78–0.93 | ||||
| >2370 DDD | 18357 | 14.2 | 3119 | 14.7 | 0.96 | 0.92–1.01 | 644 | 14.1 | 0.87 | 0.80–0.96 | ||||
| Lipophilicity | Non-user | 74109 | 57.4 | 11754 | 55.3 | 1 | 0.94 | 2693 | 58.9 | 1 | <0.001 | <0.001 | ||
| Lipophilic | 45456 | 35.2 | 7851 | 37.0 | 1.00 | 0.97–1.04 | 1567 | 34.3 | 0.88 | 0.82–0.94 | ||||
| Hydrophilic | 2844 | 2.2 | 490 | 2.3 | 1.01 | 0.92–1.12 | 77 | 1.7 | 0.70 | 0.55–0.88 | ||||
| Both | 6708 | 5.2 | 1147 | 5.4 | 0.99 | 0.92–1.05 | 232 | 5.1 | 0.87 | 0.76–1.00 | ||||
| Statin potency | Non-user | 74109 | 57.4 | 11754 | 55.3 | 1 | 0.28 | 2693 | 58.9 | 1 | <0.001 | <0.001 | ||
| Low potency | 33139 | 25.7 | 5690 | 26.8 | 1.00 | 0.97–1.04 | 1131 | 24.8 | 0.87 | 0.81–0.94 | ||||
| High potency | 7900 | 6.1 | 1414 | 6.7 | 1.05 | 0.99–1.12 | 264 | 5.8 | 0.86 | 0.75–0.98 | ||||
| Both | 13969 | 10.8 | 2384 | 11.2 | 0.98 | 0.93–1.03 | 481 | 10.5 | 0.86 | 0.78–0.96 | ||||
aAdjusted for age, sex, socioeconomic status, region, year, body mass index, smoking, alcohol, comorbidities and NSAID use.
bP-value for trend.
cP-value for the interaction between colon and rectum.
dVariables with missing data.
Analysis of other lipid lowering drugs
A significantly increased risk of CRC was observed for subjects exposed to bile acid sequestrants (OR 1.33; 95% CI: 1.12–1.57, p = 0.001), but no significant association with CRC was seen for exposures to neither fibrates nor nicotinic acid, independently on location, statin potency or dose (Supplementary Table 4).
Discussion
In this study, based on the SIDIAP database, which is representative of the Catalan population, we found a high prevalence of exposure to statins, above 40% both in cases and controls. We observed that exposure to statins was not significantly associated with the overall risk of CRC, but might be associated to a modestly reduced rectal cancer risk. For colon cancer or the combination of colon and rectal cancer (colorectal), there was no decrease in risk associated with statin use. There were no consistent associations observed for duration and cumulated dose of statin exposure and rectal cancer, while the protective association was similar for diverse statin types. Besides, exposure to acid bile sequestrants showed an increase of risk of 33%.
Previous case-control and cohort studies have suggested that statins could play a role in cancer chemoprevention12,13. However, data from clinical trials have not confirmed the protective effect seen in observational studies14. Recently, two meta-analyses and one systematic review including 40, 42, and 59 individual studies, respectively14,15,20, reported a modest reduction in risk of CRC among statin users. In contrast, previous studies that linked pharmacy and cancer registry databases found no associations between statin use and CRC risk27–34. Our study adds further evidence to the lack of a relevant effect of statins on the risk of incident CRC, supporting that any effect, if present, is of marginal magnitude. Our observation of a significant 11% increase of risk related to statin exposures shorter than 5 years is isolated, not found in other analysis such as the one exploring tumour location, and thus is of uncertain value.
Liu et al.15 showed in a stratified analyses a significant decreased association of risk in rectal cancer and for lipophilic statins, but this was limited to observational studies, and not when data was obtained from clinical trials. Our study has also observed that statin use may be selectively associated with reduced risk of rectal cancer. The reasons for this disparity in site association are unclear. Though colon and rectum are very similar at the molecular level35, environmental factors36,37 such as tobacco and physical activity38 differ in their role in their carcinogenesis. Moreover, there are clear differences among these two cancer locations regarding anatomic, embryologic, and physiologic differences39,40.
The analysis of the other lipid-lowering agents was to rule out confusion by indication, and it detected that bile acid sequestrants increased the risk of CRC, but all other lipid-lowering agents were not associated with CRC, similarly to statins. This finding was unexpected; while bile acid sequestrants are mainly used for the treatment of dyslipidaemia as they reduce low-density lipoprotein cholesterol41, they are less used than statins due to their poor tolerability (only 0.7% of our population was exposed), so that generally they are only prescribed to patients intolerant to statins or with severe dyslipidaemia. The role of bile acids on colorectal carcinogenesis has been widely studied42, and one trial had already reported a potential increase of CRC for long term use of cholestyramine back in 199243, but we have found no other references analysing the effect of these drugs on CRC, which may merit further research.
Potential limitations of this study, common to others using routinely collected data, include the lack of individual validation of exposure or cancer status. Nevertheless, SIDIAP has been widely used for other epidemiologic studies, and previous validation studies have shown that the collected information is reliable regarding disease coding21,23,44. Regarding cancer location, we found a high proportion (72%) of cases classified as “colon not specified” (C18.9), and a lower number of rectal cancers than expected (18% observed vs 48% expected45). However, the total number of cases combining colon and rectum was consistent with those expected based on the incidence data published by the Catalan cancer registries (Supplementary Fig. 1)45. While it is plausible that some rectal cancers might be coded as “colon cancer not specified”, we can assume that the specificity of the rectal cancer location should be high. Misclassification was probably independent of statin use, and although might reduce statistical power for the analysis of rectal cancer, any bias, if existent, should be towards the null hypothesis. Regarding exposure, we used actually dispensed prescriptions at pharmacies. Though there is no way to prove that dispensed prescriptions were actually consumed, data on dispensed drugs is more reliable than electronic prescriptions, which may overestimate exposure at the expense of prescriptions that are never dispensed at pharmacies. Another limitation was that we could not adjust for some risk factors for CRC like physical activity, diet or family history of CRC, because these data were not available or did not reach usable quality. Finally, we have observed in our population that a lower BMI was associated with CRC. This is a typical finding in case-control studies, because the BMI data is registered close to the cancer diagnosis, in order to objectivize weight loss caused by the CRC as a part of the diagnostic procedures, while controls may have BMI recorded more often when obesity is requiring a clinical intervention by primary care physician.
The strengths of this study include the large sample size, and the high representativeness of the population, since SIDIAP includes data on roughly 80%% of the Catalan population. Because SIDIAP contains data collected in routine practice conditions, the likelihood of observer bias is minimized. The use of electronic medical records and invoicing databases allowed us to overcome memory bias. Despite the study period was limited to 2010–2015, individual medication data was available from 2005 onwards, which allowed us to study a long period of exposure, ensuring a minimum of 5 years before the CRC diagnosis. This is of paramount importance when studying diseases with long latency such as cancer, and, in fact short exposure time is a major criticism to statin randomized trials.
In conclusion, this study adds further evidence about the lack of a relevant association between statin utilization and risk of incident CRC. While we found no association between the use of statins and overall colorectal cancer risk, the suggestive evidence of a decrease in risk for rectal cancer requires further research.
Supplementary information
Acknowledgements
The authors received funding from Bioiberica, S.A., the Agency for Management of University and Research Grants (AGAUR) of the Catalan Government grant 2017SGR723, also from the Instituto de Salud Carlos III, co-funded by FEDER funds –a way to build Europe– grant PI14-00613.
Author Contributions
Study conception and design: Victor Moreno, Gemma Ibáñez, Caridad Pontes, Rosa Morros and Mª Ángeles Quijada-Manuitt. Statistical analysis: Elisabet Guinó. Interpretation of the data and drafting of the manuscript: Gemma Ibáñez-Sanz, Caridad Pontes, Rosa Morros, Elisabet Guinó and Victor Moreno. Contributions to interpretation of the data and drafting the final version of the manuscript were made by all authors, including María Aragón, Luisa de la Peña, Marga Domínguez, Lorena Rodríguez-Alonso, Àlex Blasco, and Ana García-Rodríguez. All authors approved the final version of the manuscript.
Data Availability
The datasets generated and analysed during the current study are not publicly available due to restrictions imposed by the data provider (the Information System for Development of Primary Care Research, SIDIAP) but researchers interested can contact SIDIAP (www.sidiap.org) to propose a research project based on their database.
Competing Interests
The corresponding authors (V.M., R.M.) have received funding from Bioiberica, S.A., Barcelona to study epidemiological evidence of drugs associated to CRC. The authors acted with complete independence of this company that has no statins or lipid lowering drug in their portfolio.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rosa Morros, Email: rmorros@idiapjgol.org.
Victor Moreno, Email: v.moreno@iconcologia.net.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49877-5.
References
- 1.Ferlay, J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet], http://globocan.iarc.fr (2013).
- 2.Arnold M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund International/American Institute for Cancer Resarch. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer (2017).
- 4.Alberts DS, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 5.Schatzkin A, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342:1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 6.Martinez ME, et al. Design and baseline characteristics of study participants in the Wheat Bran Fiber trial. Cancer Epidemiol Biomarkers Prev. 1998;7:813–816. [PubMed] [Google Scholar]
- 7.Heine-Broring RC, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer. 2015;136:2388–2401. doi: 10.1002/ijc.29277. [DOI] [PubMed] [Google Scholar]
- 8.Baron JA, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med. 2015;373:1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostom A, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 10.Flossmann E, Rothwell PM. British Doctors Aspirin, T. & the, U. K. T. I. A. A. T. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 11.Salvo F, et al. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–866. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 12.Katz MS. Therapy insight: Potential of statins for cancer chemoprevention and therapy. Nat Clin Pract Oncol. 2005;2:82–89. doi: 10.1038/ncponc0097. [DOI] [PubMed] [Google Scholar]
- 13.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 14.Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol. 2014;20:1858–1870. doi: 10.3748/wjg.v20.i7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014;25:237–249. doi: 10.1007/s10552-013-0326-6. [DOI] [PubMed] [Google Scholar]
- 16.Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol. 2016;45:71–81. doi: 10.1016/j.canep.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Lochhead, P. & Chan, A. T. Statins and colorectal cancer. Clin Gastroenterol Hepatol11, 109–118; quiz e113–104 (2013). [DOI] [PMC free article] [PubMed]
- 18.Agarwal B, et al. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 1999;5:2223–2229. [PubMed] [Google Scholar]
- 19.Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22:1061–1067. doi: 10.1093/carcin/22.7.1061. [DOI] [PubMed] [Google Scholar]
- 20.Dobrzycka, M. et al. Statins and Colorectal Cancer - A Systematic Review. Exp Clin Endocrinol Diabetes [Epub ahead of print] (2018). [DOI] [PubMed]
- 21.Bolibar B, et al. [SIDIAP database: electronic clinical records in primary care as a source of information for epidemiologic research] Med Clin (Barc) 2012;138:617–621. doi: 10.1016/j.medcli.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Guidelines for ATC Classification and DDD Assignment, https://www.whocc.no/atc_ddd_index (2018).
- 23.Ramos R, et al. Rev Esp Cardiol (Engl Ed) 2012. Validity for use in research on vascular diseases of the SIDIAP (Information System for the Development of Research in Primary Care): the EMMA study; pp. 29–37. [DOI] [PubMed] [Google Scholar]
- 24.Galceran, J. & Marcos, R. Cancer Incidence in Catalonia (2008–2012), http://ci5.iarc.fr (2017).
- 25.Bytyci I, et al. Hydrophilic vs lipophilic statins in coronary artery disease: A meta-analysis of randomized controlled trials. J Clin Lipidol. 2017;11:624–637. doi: 10.1016/j.jacl.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez-Berjon MF, et al. Constructing a deprivation index based on census data in large Spanish cities (the MEDEA project) Gac Sanit. 2008;22:179–187. doi: 10.1157/13123961. [DOI] [PubMed] [Google Scholar]
- 27.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friis S, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 30.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 2011;11:409. doi: 10.1186/1471-2407-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haukka J, et al. Incidence of cancer and statin usage–record linkage study. Int J Cancer. 2010;126:279–284. doi: 10.1002/ijc.24536. [DOI] [PubMed] [Google Scholar]
- 32.Singh H, et al. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol. 2009;104:3015–3023. doi: 10.1038/ajg.2009.574. [DOI] [PubMed] [Google Scholar]
- 33.Cheng MH, et al. Statin use and the risk of colorectal cancer: a population-based case-control study. World J Gastroenterol. 2011;17:5197–5202. doi: 10.3748/wjg.v17.i47.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung HW, Chan AL, Lo D, Leung JH, Chen HL. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert Opin Drug Saf. 2013;12:19–27. doi: 10.1517/14740338.2013.744392. [DOI] [PubMed] [Google Scholar]
- 35.Sanz-Pamplona R, et al. Gene expression differences between colon and rectum tumors. Clin Cancer Res. 2011;17:7303–7312. doi: 10.1158/1078-0432.CCR-11-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng J, et al. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur J Cancer Prev. 2015;24:6–15. doi: 10.1097/CEJ.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 37.World Cancer Research Fund International/American Institute for Cancer Research. Continous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer (2017).
- 38.Wei EK, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10:219–229. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 41.Scaldaferri F, Pizzoferrato M, Ponziani FR, Gasbarrini G, Gasbarrini A. Use and indications of cholestyramine and bile acid sequestrants. Intern Emerg Med. 2013;8:205–210. doi: 10.1007/s11739-011-0653-0. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen TT, Ung TT, Kim NH, Jung YD. Role of bile acids in colon carcinogenesis. World J Clin Cases. 2018;6:577–588. doi: 10.12998/wjcc.v6.i13.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Lipid Research Clinics Investigators The Lipid Research Clinics Coronary Primary Prevention Trial. Results of 6 years of post-trial follow-up. Arch Intern Med. 1992;152:1399–1410. doi: 10.1001/archinte.1992.00400190041009. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Gil Mdel M, et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP) Inform Prim Care. 2011;19:135–145. doi: 10.14236/jhi.v19i3.806. [DOI] [PubMed] [Google Scholar]
- 45.Galceran J, et al. Cancer incidence in Spain, 2015. Clin Transl Oncol. 2017;19:799–825. doi: 10.1007/s12094-016-1607-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to restrictions imposed by the data provider (the Information System for Development of Primary Care Research, SIDIAP) but researchers interested can contact SIDIAP (www.sidiap.org) to propose a research project based on their database.