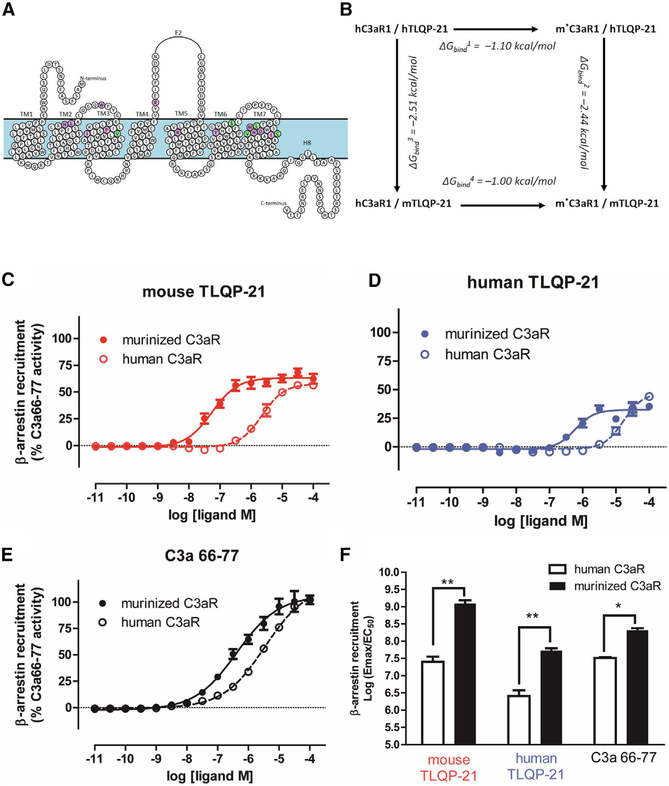
Figure 5. Murinizing the hC3aR1 by Site-Directed Mutagenesis Enhances TLQP-21 Binding Affinity and Potency.
(A) Murinized hC3aR1 (m*C3aR1) from the homology model, highlighting conserved amino acids (purple circles) and amino acids affected by variants in rodents (V103I, S400L, L413V, V419M, and C420S, green circles).
(B) Change in binding affinity (ΔGbind) of mouse and human TLQP-21 to hC3aR1 and m*C3aR1 was calculated based on the mutagenesis thermodynamic cycle.
(C-E) β-Arrestin recruitment Tango assay transfected with either hC3aR1 or m*C3aR1 and incubated with a range of concentrations with either mTLQP-21 (hC3aR1, EC50 = 2.3 μM; m*C3aR1, EC50 = 0.055 μM) (C), hTLQP-21 (hC3aR1, EC50 = 16.9 μM; m*C3aR1, EC50 = 0.59 μM) (D), or C3a63–77 (hC3aR1, EC50 = 3.6 μM; m*C3aR1, EC50 = 0.4 μM) (E). Data represent mean and SE of triplicates from three independent experiments.
(F) Quantitative comparison of β-arrestin recruitment functional activity as measured by log(Emax/EC50) from three independent experiments in (C)–(E) (ANOVA F(5,12) = 2.5, p = 0.032). *p < 0.05, **p < 0.01 comparing m*C3aR1 with hC3aR1. Emax, maximal effect at high drug concentrations when all the receptors are occupied by the drug. Data are expressed as average and SEM.