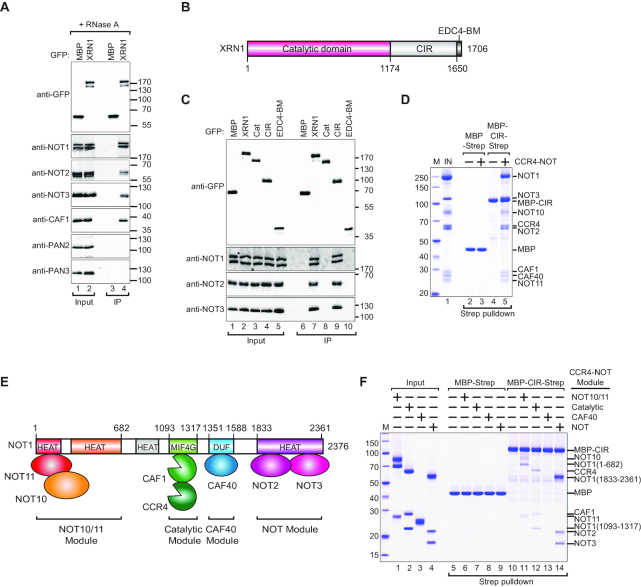
Figure 1.
XRN1 interacts directly with CCR4–NOT via a low-complexity region. (A) The interaction of GFP-tagged XRN1 with endogenous deadenylation factors. The proteins were immunoprecipitated using anti-GFP antibodies. GFP-MBP served as a negative control. Inputs (10%) and bound fractions (IP; 30%) were analyzed by western blotting using the indicated antibodies. The size markers (kDa) are shown on the right of the panel. (B) The domain organization of human XRN1. XRN1 consists of a catalytic domain (indicated in red) and a C-terminal variable, low-complexity region comprising the C-terminal interacting region (CIR, light gray) and the EDC4-binding motif (EDC4-BM, dark gray). The residue numbers are indicated at the domain boundaries. (C) The interaction of XRN1 (full length or the indicated fragments) with the endogenous CCR4–NOT subunits. The proteins were immunoprecipitated using anti-GFP antibodies and analysed as described in (A). (D) A streptavidin pulldown assay showing the interaction of Strep-tagged CIR with the recombinant purified CCR4–NOT. Strep-tagged MBP served as a negative control. The size markers (kDa) denoted by ‘M’ are shown on the left of the panel. (E) Schematic representation of the CCR4–NOT complex and subcomplex modules. (F) A streptavidin pulldown assay showing the interaction of Strep-tagged CIR with recombinant purified CCR4–NOT modules. Strep-tagged MBP served as a negative control.