Abstract
T-cell activation is mediated by a combination of signals from the antigen receptor (TCR) and co-receptors such as CD28, cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death antigen 1 (PD-1), CD28H and others. Each is a member of the CD28 receptor gene family. CD28 sends positive signals that promote T-cell responses, while CTLA-4 and PD-1 limit responses. It is the balance between these positive and negative signals that determines the amplitude and level of T-cell responses. The regulatory role of other family members is also becoming the focus of increasing interest. The function of certain CD28 family members such as CTLA-4 and PD-1 is dependent the expression of CD28. Together, these findings have important implications in generation of immune responses and the application of anti-receptor blocking reagents in immunotherapy.
Keywords: Co-receptors, CD28, CTLA-4, PD-1, CD28H
Introduction
Co-receptors of the CD28 family of receptors
The co-receptor CD28 defines a family of immunoglobulin-like co-receptors and ligands involved in the regulation of the immune response. These include CD28, inducible co-stimulator (ICOS), cytotoxic T-lymphocyte antigen-4 (CTLA-4), and programmed cell death protein 1 (PD-1) (Figure 1, upper panel) [1]. Both CD28 and CTLA-4 use their signature MYPPPY binding motifs to competitively bind ligands, CD80 (B7-1) and CD86 (B7-2) [2]. CD80 binds CTLA-4 and CD28 with different affinities (Kd values of approximately 12 and 200 nM, respectively) [3]. The higher affinity CTLA-4 binding is due to an arrangement in which bivalent homodimers bridge bivalent CD80 molecules [4]. T-cells from CD28-deficient mice show reduced proliferation in response to peptide antigens [5, 6]. Although high strength T-cell receptor (TCR) signaling with high avidity peptide can activate T-cells, co-ligation with CD28 is required to provide complementary signals for activation. TCR alone often results in anergy, or cell death [7, 8]. Repeated antigen stimulation or long-term viral infection can bypass the requirement for CD28 [9]. CD28 co-signals can stabilize cytokine messenger RNA (mRNA) [10] and play a role on cell metabolism [11.
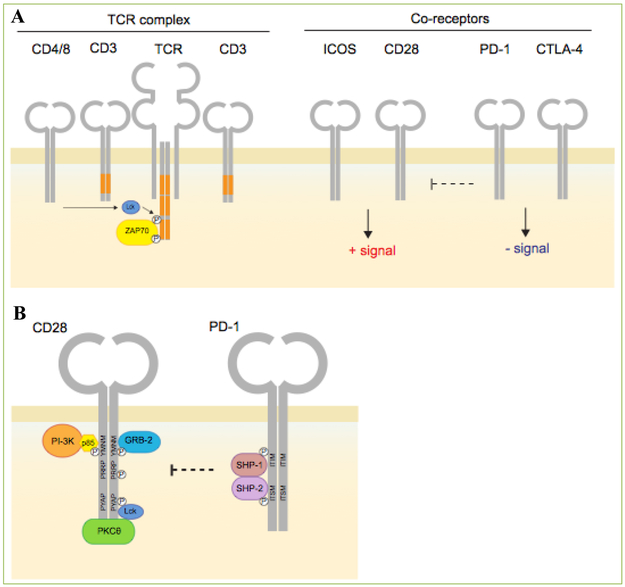
Figure 1. CD28 family of receptors and an interplay between PD-1 and CD28. Upper:
Diagram showing the structure of the antigen-receptor (TCR) and co-receptors CD28, ICOS, PD-1 and CTLA-4 on T-cells. CD4/CD8-p56lck phosphorylates the TCR leading to the recruitment of ZAP-70. CD28 and ICOS send positive signals while PD-1 and CTLA-4 dampen the immune response. Lower: Illustration showing the interplay between PD-1 and CD28 at the level of signaling. p56lck phosphorylates tyrosine residues on PD-1 and CD28 which allows CD28 to recruit PI 3K and GRB2, whereas PD-1 recruits the phosphatases SHP-2 (Shp2) and SHP-1 (Shp1). PI 3K binds to the CD28 pYMNM motif, while the SHP-2 (Shp2) binds to the ITIM and ITSM tyrosine motifs in PD-1. SHP2 showed a marked preference in dephosphorylating CD28 (including the pYMNM motif) relative to other substrates such as the TCRzeta chain and the co-receptor ICOS.
While the cytoplasmic domain of CD28 lacks intrinsic catalytic activity, it possesses key motifs that bind to SH2 and SH3 domains of signaling proteins. The src-family kinases p56lck and p59fyn phosphorylate tyrosine residues on CD28 [12]. Phosphorylated YMNM binds to the SH2 domains of phosphatidyl inositol 3 kinase (PI3K) and Grb2 [13, 14]. PI3K generates the lipids PIP2 and PIP3 that bind the pleckstrin homology (PH) domains within proteins such as phosphoinositide-dependent protein kinase 1 (PDK1), which in turn activates protein kinase B (PKB/AKT). The YXNX sequence of the YMNM motif binds adapter protein Grb2 [13, 15] which in turn binds exchange factor for Son of Sevenless for the activation of GTPase p21ras. The loss of Grb2 binding by mutation of the asparagine (N) residue leads to a loss of CD28-mediated phosphorylation of the guanine nucleotide exchange factor Vav1 and the activation of the serine/threonine kinase c-Jun kinase (JNK) [16].
CTLA-4
Unlike CD28, CTLA-4 dampens T-cell responses in a manner that can protect against the development of auto-proliferative or autoimmune disease [2, 17, 18, 19] CTLA-4-deficient (Ctla-4−/−) mice show a profound hyper-proliferative phenotype leading to death within 3 weeks of age due to massive tissue infiltration and organ destruction [6]. Antibody cross-linking is also inhibitory [20], although it is unclear whether there is a straightforward connection between these two sets of observations. Anti-CTLA-4 binds to PI3K and can activate the JNK pathway [21, 22], while concurrently inhibiting T-cell activation. CTLA-4 mediates effects via cell intrinsic and extrinsic signals [23, 24] In one model, CTLA-4 induces motility that limits contact between conventional T-cells and dendritic cells [25]. Regulatory T-cells (Tregs) are resistant to this effect [26], allowing for CTLA-4 blockade of CD80/CD86 and/or removal of a portion from the cell surface [27].
PD-1
Like CTLA-4, PD-1 is a member of the CD28 gene family that is expressed on T-cells in response to activation [1, 28]. It binds to novel ligands PDL-1 (B7H-1) and PDL-2 (B7H-2) that are expressed on hematopoetic and non-hematopoetic cells. Unlike the founding member of the family, which generates positive signals that complement T-cell receptor (TCR) signaling, PD-1 produces negative signals that limit T-cell proliferation and effector functions. PD-1 inhibitory function is particularly evident in hypo-responsive ‘exhausted T-cells’ that develop with chronic viral infections and repeated antigen stimulation. Blocking antibodies against PD-1 can restore functionality to these T-cells leading to viral clearance[29, 30, 31, 32].
The cytoplasmic tail of PD-1 contains two other structural motifs, an ITIM (immunoreceptor tyrosine-based inhibition, i.e. VDYGEL) motif, followed by an ITSM (immunoreceptor tyrosine-based switch, i.e. TEYSEV) motif [33] (Figure 1, lower panel). These motifs bind to the Src homology region 2 domain-containing phosphatase-1 (SHP-1) [also PTPN6] and related SHP-2 [PTPN11]. Both phosphatases bind to PD-1 on immune cells, although the negative signaling has been attributed to SHP-2 binding to the ITSM motif [1, 28, 34]. By contrast, while CTLA-4 may associate with phosphatases, it lacks conventional binding sites, and inhibits via cell intrinsic and extrinsic mechanisms [24].
Other family members of CD28/B7 family
Several new members of the CD28/B7 family have recently been identified. The CD28 homologue (CD28H) [37] is one such co-receptor. It is also known as the transmembrane and immunoglobulin domain-containing protein 2 variant 2 (TMIGD2) [35] ]or immunoglobulin-containing and proline-rich receptor-1 (IGPR-1) [36] and shares 10% sequence homology with CD28, ICOS, CTLA-4 and PD-1 [37]. CD28H is expressed in thymus, lung, heart and kidney [36] and it is constitutively expressed in all naive T-cells as well as NK cells, and is down-regulated following repeated in-vitro stimulation. Interestingly, while CD28H orthologues are present in various species, it is not present in laboratory mice or rats [37]. The extracellular domain of CD28H contains an IgV-like domain and its cytoplasmic tail contains a proline rich motif that has been shown to interact with the SH3 domains of various proteins including SPIN90, CACNB2 and BPAG1. Initial studies identified CD28H as an adhesion molecule involved in cell-cell interaction, cell migration and angiogenesis [36, 38].
The ligand for CD28H has been identified as HERV-H LTR-associating 2 (HHLA2) renamed B7H7 [37, 39]. Similar to CD28H, B7H7 orthologues are not present in laboratory mice or rats [40]. B7H7 possesses an IgV-IgC-IgV domain in its extracellular region [39], and is expressed in intestines, breast, kidney, gallbladder, placenta and B cells [37, 40]. B7H7 is believed to interact with CD28H via its first IgV domain and to send a co-stimulatory signal [37], or co-inhibitory signal in the presence or absence of CD28H expression [38, 41]. Aberrant expression of B7H7 has been observed in various cancer types including lung [42] osteosarcoma [43] and pancreatic cancers [44]. Other recently identified B7 family members whose overexpression correlate with poor cancer prognosis include B7H4 (B7x, B7S1, VTCN1) and B7H5 (PD-1H, VISTA, GI24, DIES1). Receptors have yet to be identified for these ligands and their functions and involvements in disease warrants further investigation.
Immunotherapy
The past few years have witnessed breakthroughs in immune check-point blockade for the treatment of various human cancers. This was first observed with antibody blockade of the negative co-receptor CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) (i.e. using Ipilimumab) and was followed by blockade of a second key co-receptor, programmed cell death-1 (PD-1) (i.e. Nivolumab and Pembrolizumab), or its ligand (PD-L1) (i.e. Atezolizumab), either alone, or in combination with anti-CTLA-4 [45].
Recent data indicates that CD28 can interface with negative co-receptors, either via extracellular or intracellular pathways. In the case of CTLA-4, studies have shown that autoimmune disease in Ctla4−/− (i.e. CTLA-4) mice depend on CD28 expression [46]. More recently, two new papers have shown that PD-1 operates via inhibition of CD28 signaling [47, 48, 49]. PD-1 associates with SHP-2 which preferentially dephosphorylates CD28 (Figure 1B). The preferred de-phosphorylation of CD28 was observed in assays for reduced overall phosphorylation and PI3K SH2 domain binding to the CD28 pYMNM motif. Little de-phosphorylation of the T-cell receptor or related ICOS was observed. Both PD-1 and CD28 preferentially co-cluster to provide a mechanism by which these co-receptors interact for de-phosphorylation. Consistent with this, others have shown that PD-1 blockade requires the expression of CD28 for the recovery of exhausted T cell responses [48]. The interplay involving CTLA-4 and PD-1 with CD28 may be indicative of a general theme of interplay between members of the CD28 family in the regulation of T-cell responses.
Conclusions
The CD28/B7 family of receptors and ligands are involved in the regulation of the immune responses. Certain co-receptors such as CD28 and ICOS send positive signals while others such as CTLA-4 and PD-1 lead to a dampening of the immune response. Recent evidence indicates that this family of co-receptors interact and cross-regulate each other in regulating T-cell responses.
Acknowledgement
This work was supported by NIH R01 (No. AI049466 to Fadi Lakkis and Christopher E. Rudd).
Footnotes
Conflicting interests
The authors have declared that no conflict of interests exist.
References
- 1.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 2016; 34:539–573. [DOI] [PubMed] [Google Scholar]
- 2.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev 2009; 229:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med 1997; 185:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 2001; 410:608–611. [DOI] [PubMed] [Google Scholar]
- 5.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science 1993; 261:609–612. [DOI] [PubMed] [Google Scholar]
- 6.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995; 3:541–547. [DOI] [PubMed] [Google Scholar]
- 7.Linsley PS Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule-4 receptors during T cell activation? J Exp Med 1995; 182:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol 1993; 11:191–212. [DOI] [PubMed] [Google Scholar]
- 9.Kundig TM, Shahinian A, Kawai K, Mittrucker HW, Sebzda E, Bachmann MF, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 1996; 5:41–52. [DOI] [PubMed] [Google Scholar]
- 10.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today 1990; 11:211–216. [DOI] [PubMed] [Google Scholar]
- 11.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002; 16:769–777. [DOI] [PubMed] [Google Scholar]
- 12.Raab M, Cai YC, Bunnell SC, Heyeck SD, Berg LJ, Rudd CE. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc Natl Acad Sci U S A 1995; 92:8891–8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider H, Cai YC, Prasad KV, Shoelson SE, Rudd CE. T cell antigen CD28 binds to the GRB-2/SOS complex, regulators of p21ras. Eur J Immunol 1995; 25:1044–1050. [DOI] [PubMed] [Google Scholar]
- 14.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol 2003; 3:544–556. [DOI] [PubMed] [Google Scholar]
- 15.Schneider H, Cai YC, Cefai D, Raab M, Rudd CE. Mechanisms of CD28 signalling. Res Immunol 1995; 146:149–154. [DOI] [PubMed] [Google Scholar]
- 16.Kim HH, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J Biol Chem 1998; 273:296–301. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol 2002; 14:391–396. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity 2001; 14:145–155. [DOI] [PubMed] [Google Scholar]
- 19.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997; 7:885–895. [DOI] [PubMed] [Google Scholar]
- 20.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 1996; 183:2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider H, Mandelbrot DA, Greenwald RJ, Ng F, Lechler R, Sharpe AH, et al. Cutting edge: CTLA-4 (CD152) differentially regulates mitogen-activated protein kinases (extracellular signal-regulated kinase and c-Jun N-terminal kinase) in CD4+ T cells from receptor/ligand-deficient mice. J Immunol 2002; 169:3475–3479. [DOI] [PubMed] [Google Scholar]
- 22.Oosterwegel MA, Greenwald RJ, Mandelbrot DA, Lorsbach RB, Sharpe AH. CTLA-4 and T cell activation. Curr Opin Immunol 1999; 11:294–300. [DOI] [PubMed] [Google Scholar]
- 23.Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol 11:203–210. [DOI] [PubMed] [Google Scholar]
- 24.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol 2008; 8:153–160. [DOI] [PubMed] [Google Scholar]
- 25.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006; 313:1972–1975. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Schneider H, Rudd CE. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood 2012; 120:4560–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi OS, Zheng Y, Nakamura K, Attridhe K, Manzotti C, Schmidt EM, et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell Extrinsic Function of CTLA-4. Science 2011; 332:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013; 14:1212–1218. [DOI] [PubMed] [Google Scholar]
- 29.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell 2009; 138:30–50. [DOI] [PubMed] [Google Scholar]
- 30.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009; 458:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Audert RD, Sharpe AH, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med 2008. 205:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansell SM, Lesokhin AM, Borello I, Halwani A, Scott E, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 1994; 23:04–706. [DOI] [PubMed] [Google Scholar]
- 34.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004; 173:945–954. [DOI] [PubMed] [Google Scholar]
- 35.Clark HF, Gumey AL, Abaya E, Baker K, Baldwin D, Brush J, et al. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res 2003; 13:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. IGPR-1 Is Required for Endothelial Cell-Cell Adhesion and Barrier Function. Mol Biol Cell 2012; 23:1645–1656. [Google Scholar]
- 37.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun 2013; 4:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YH, Meyer RD, Bondzie PA, Jiang Y, Rashimi I, Rezazadeh K, et al. IGPR-1 Is Required for Endothelial Cell-Cell Adhesion and Barrier Function. J Mol Biol 2016; 428:5019–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flajnik MF, Tlapakova T, Criscitiello MF, Krylov V, Ohta Y. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7's historical relationship with the MHC. Immunogenetics 2012; 64:571–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janakiram M, Fineberg S, Fiser A, Montagna C, Medavarapu R, Castano E, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res 2015; 21:2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A 2013; 110:9879–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, Janakiram M Borczuk A, Lin J, Qiu W, Liu H, et al. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin Cancer Res 2017; 23:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koirala P, Roth M, Gill J, Chinai JM, Ewart MR, Piperdi S, et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep 2016; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byers JT, Paniccia A, Kaplan J, Koenig M, Kahn N, Wilson L, et al. Expression of the novel costimulatory molecule B7-H5 in pancreatic cancer. Ann Surg Oncol 2015; 3:1574–1579. [DOI] [PubMed] [Google Scholar]
- 45.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011; 11:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4 / mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci U S A 2007; 104:13756–13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017; 355:1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamphorst AO, Wieland A, Nasti T, Yang s, Zhang R, Barber DL,. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017; 355:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krueger J, Rudd CE. Two Strings in One Bow: PD-1 Negatively Regulates via Co-receptor CD28 on T Cells. Immunity 2017; 46:529–531. [DOI] [PubMed] [Google Scholar]