Figure 1.
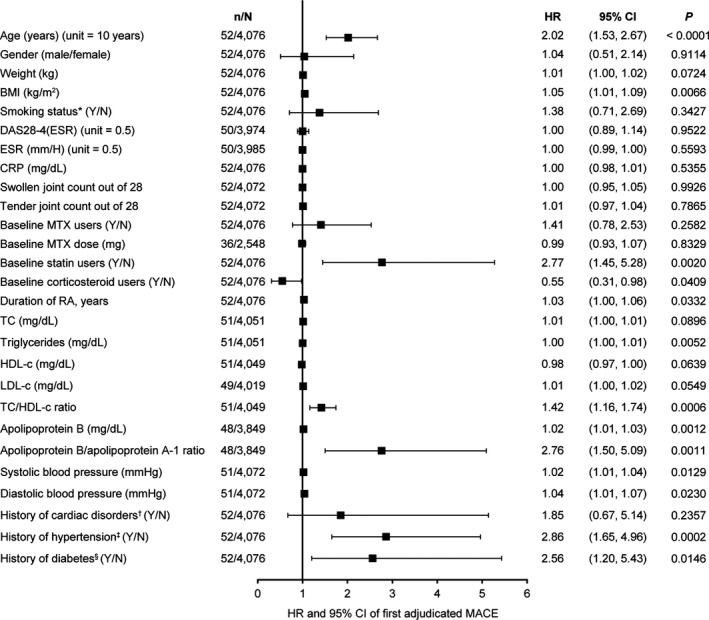
Univariable analyses of associations between baseline variables and the occurrence of major adverse cardiovascular events (MACE) during tofacitinib treatment. For all continuous variables, unit = 1 unless specified otherwise; in unit = x, “x” is the change in the continuous variable corresponding to which the change in hazards is observed. * = smoking status as recorded at baseline; † = including any of the Medical Dictionary for Regulatory Activities–coded terms: angina pectoris, arrhythmia, atrial flutter, atrial fibrillation, first‐degree atrioventricular block, left bundle branch block, cardiac failure, cardiac failure congestive, cardiac valve disease, cardiomegaly, cardiomyopathy, coronary artery disease, hypertensive cardiomyopathy, left ventricular hypertrophy, mitral valve incompetence, mitral valve prolapse, valve prolapse, myocardial infarction, palpitations, sinus bradycardia, sinus tachycardia, tachycardia, tachycardia paroxysmal, tricuspid valve incompetence, and ventricular extrasystole; ‡ = based on prior medical history; § = based on both use of diabetes medication and medical history. n = number of patients with future MACE for each baseline variable; N = number of patients included in the analysis for each baseline variable; HR = hazard ratio; 95% CI = 95% confidence interval; BMI = body mass index; DAS28‐4 (ESR) = 4‐variable Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; CRP = C‐reactive protein; MTX = methotrexate; RA = rheumatoid arthritis; TC = total cholesterol; HDL‐c = high‐density lipoprotein cholesterol; LDL‐c = low‐density lipoprotein cholesterol.
