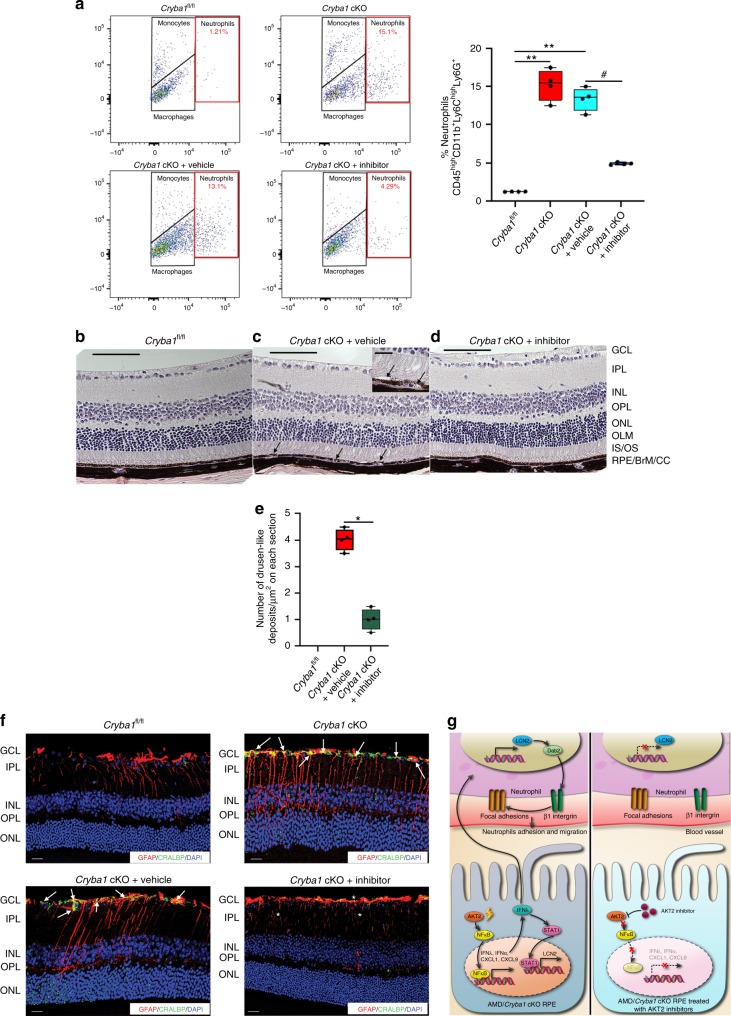
Fig. 8.
Inhibiting AKT2 phosphorylation blocks neutrophil infiltration into the retina and rescues early RPE changes in Cryba1 cKO mice. a Flow cytometry dot plots denoting monocytes, macrophages and neutrophils from mouse retina (as explained in Fig. 1a). The neutrophil population (%CD45highCD11b+Ly6ChighLy6G+ cells, red gated) significantly increased in the 12 month Cryba1 cKO mouse retina ± intravitreal vehicle treatment, compared to age-matched Cryba1fl/fl (control). Intravitreal treatment with the AKT2 inhibitor (CCT128930) significantly reduced neutrophils in cKO retina. Graphs denote % CD45highCD11b+Ly6ChighLy6G+ cells. n = 4. **P < 0.01 and #P < 0.05 (one-way ANOVA and post hoc test). b–d Representative histological sections (H&E) of retina from 1 year old Cryba1fl/fl mouse, showing normal structure (b). Age-matched Cryba1 cKO mouse (c) intravitreally injected with vehicle (2.5% DMSO in PBS) shows RPE and photoreceptor lesions with pigmentation changes (arrows). Inset in c, shows higher magnification of RPE lesions indicating possible debris accumulation between Bruch’s membrane and RPE and separation of photoreceptors from RPE (arrows). In contrast, inhibitor (CCT128930, inhibits AKT2 activation) treated Cryba1 cKO mice (d), exhibited normal structure after 4 weeks. e Bar graph showing decrease in number of sub-retinal drusen-like deposits after AKT2 inhibitor treatment compared to vehicle-treated cKO mice. n = 4. Scale bars, 100 and 50 μm (inset). *P < 0.05 (one-way ANOVA and post hoc test). f Retina sections from 12-month-old Cryba1fl/fl or Cryba1 cKO mice stained with glial fibrillary acidic protein (GFAP, red) and cellular retinaldehyde-binding protein (CRALBP, green). Sections from cKO mice ± intravitreal vehicle showed extensive staining of the Müller glial processes (cells staining for both CRALBP and GFAP, yellow indicating activation, arrows). This was significantly reduced after inhibitor treatment (asterisk). n = 4. Scale Bar, 50 μm. g Schematic depicting neutrophils homing into the retina and releasing LCN-2, generating pro-inflammatory conditions that contribute to elements of early AMD pathobiology. Our data suggest that IFNλ triggers transmigration of neutrophils into the retina through activation of the LCN-2/Dab2/integrin β1 signaling axis (Left panel). Inhibiting AKT2-dependent signaling can neutralize inflammatory signals and block neutrophil infiltration (Right Panel). Thus, AKT2 inhibitors should be assessed as potential therapy at the earliest stages of AMD