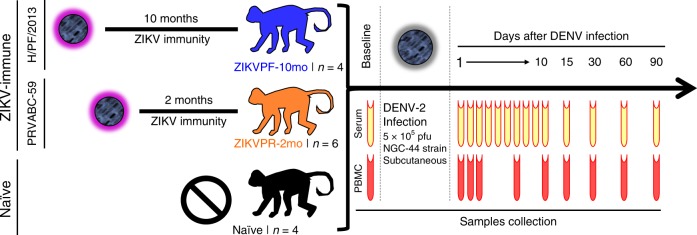
Fig. 1.
Experimental design for DENV-2 challenge of ZIKV-immune and naive macaques. Fourteen young adult male rhesus macaques (Macaca mulatta), matched in age and weight, were divided into three cohorts. ZIKVPF-10mo (n = 4): composed of four animals (5K6, CB52, 2K2, and 6N1) that were inoculated with 1 × 106 pfu/500 µl of the ZIKV H/PF/2013 strain subcutaneously 10 months before (middle convalescence) DENV-2 challenge. ZIKVPR-2mo (n = 6): composed of six animals (MA067, MA068, BZ34, MA141, MA143, and MA085) that were inoculated with 1 × 106 pfu/500 µl of the contemporary ZIKV PRVABC59 strain 2 months before (early convalescence) DENV-2 challenge. Both ZIKV strains used for previous exposure of these groups are > 99.99% comparable in amino acid identity (Supplementary Table 1). Naive (n = 4): composed of four ZIKV/DENV naive animals (MA123, MA023, MA029, and MA062) as a control group. Prior to DENV-2 challenge, all animals were subjected to quarantine period. All cohorts challenged subcutaneously (deltoid area) with 5 × 105 pfu/500 µl of DENV-2 New Guinea 44 strain (NGC44). After DENV-2 challenge, all animals were extensively monitored for evidence of disease and clinical status by vital signs, such as external temperature (°C), weight (Kg), CBC, and CMP panels at the Caribbean Primate Research Center (CPRC). Blood samples were collected at baseline, 1–10, 15, 30, 60, and 90 days after DENV infection. In all timepoints, the blood samples were used for serum separation (yellow). PBMCs isolation (red) was performed in different tubes with citrate as anticoagulant at baseline, 1, 2, 3, 7, 10, 15, 30, 60, and 90 days after DENV infection