Fig. 7.
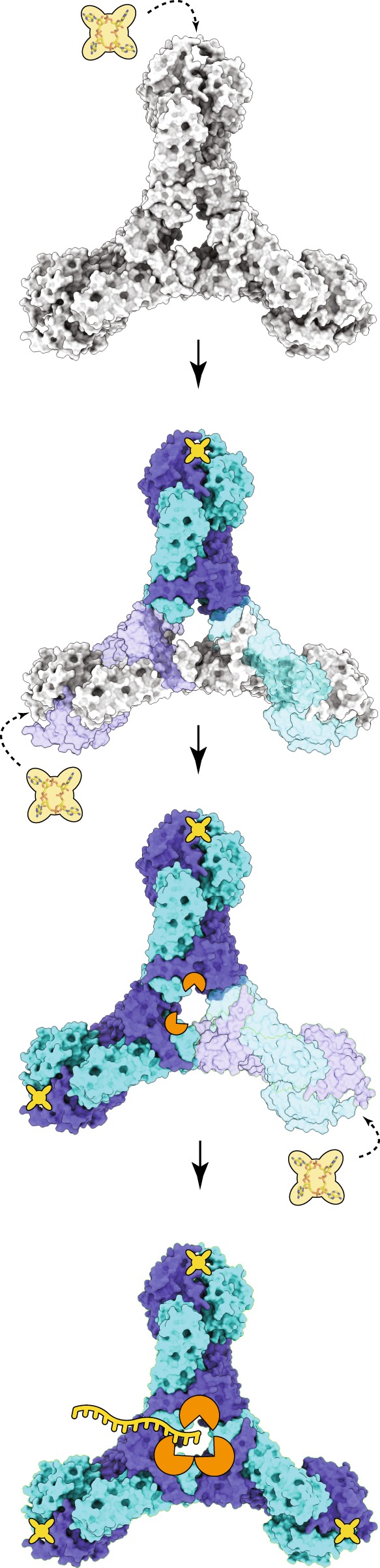
Model for SisCsx1 RNase catalysis activation by cOA4. The binding of cOA4 (tetralobal shape) by the CARF domains fully activates the first dimer (strong blue and strong sky blue) and induces a conformational change through the HTH to the HEPN domains of two adjacent monomers (light blue and light sky blue) favoring cOA4 binding in any of their available cOA4 binding sites. The binding of the second cOA4 molecule fully activates the second dimer (strong blue and strong sky blue), promoting some initial RNase activity and triggering conformational changes in the two monomers of the third dimer (light blue and light sky blue) leading to the binding of the third cOA4 molecule and the full RNase activation
