Figure 3.
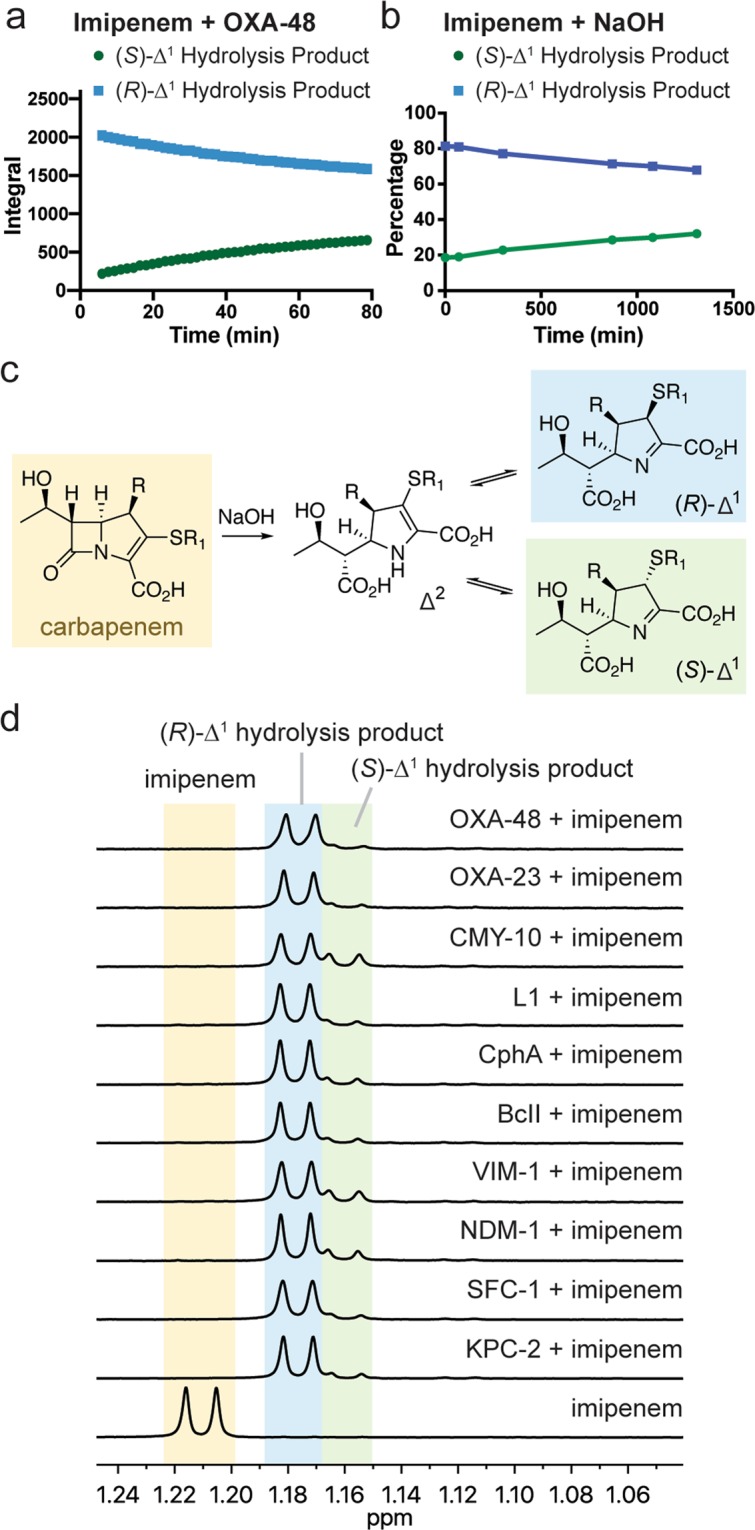
Stereoselectivity of enzymatic and non-enzymatic carbapenem hydrolysis. (a) NMR time course showing the levels of the (R)-Δ1 and (S)-Δ1 hydrolysis products formed by OXA-48 (5 µM) and imipenem (1 mM). Imipenem was fully hydrolysed by the first NMR measurement. (b) NMR time course showing the levels of the (R)-Δ1 and (S)-Δ1 hydrolysis products formed by treatment of imipenem (10 mM) with NaOH (100 mM). Imipenem was fully hydrolysed by the time of the first NMR measurement. (c) Scheme showing hydroxide-mediated degradation of a carbapenem, which is expected to initially form the Δ2 hydrolysis product. Rapid tautomerisation is then proposed to occur, with kinetically controlled protonation, forming the (R)-Δ2 hydrolysis product. Over time, tautomerisation and epimerisation occurs, forming the (S)-Δ2 hydrolysis product. (d) NMR spectra (600 MHz) showing the apparent preferential formation of the (R)-Δ1 hydrolysis product from imipenem (1 mM) by class A (KPC-2, SFC-1), class B (NDM-1, VIM-1, BcII, CphA, L1) class C (CMY-10), and class D (OXA-23, OXA-48) β-lactamases (5 µM, apart from CphA and L1, which were 1 µM and 0.15 µM, respectively). All spectra were acquired 5 min after mixing enzyme and imipenem, apart from CMY-10, which was acquired after 250 min. Note that the observations described in panel b suggest that the Δ2 imipenem hydrolysis product may be the nascent enzymatic product; this undergoes rapid tautomerisation giving the (R)-Δ1 hydrolysis product.
