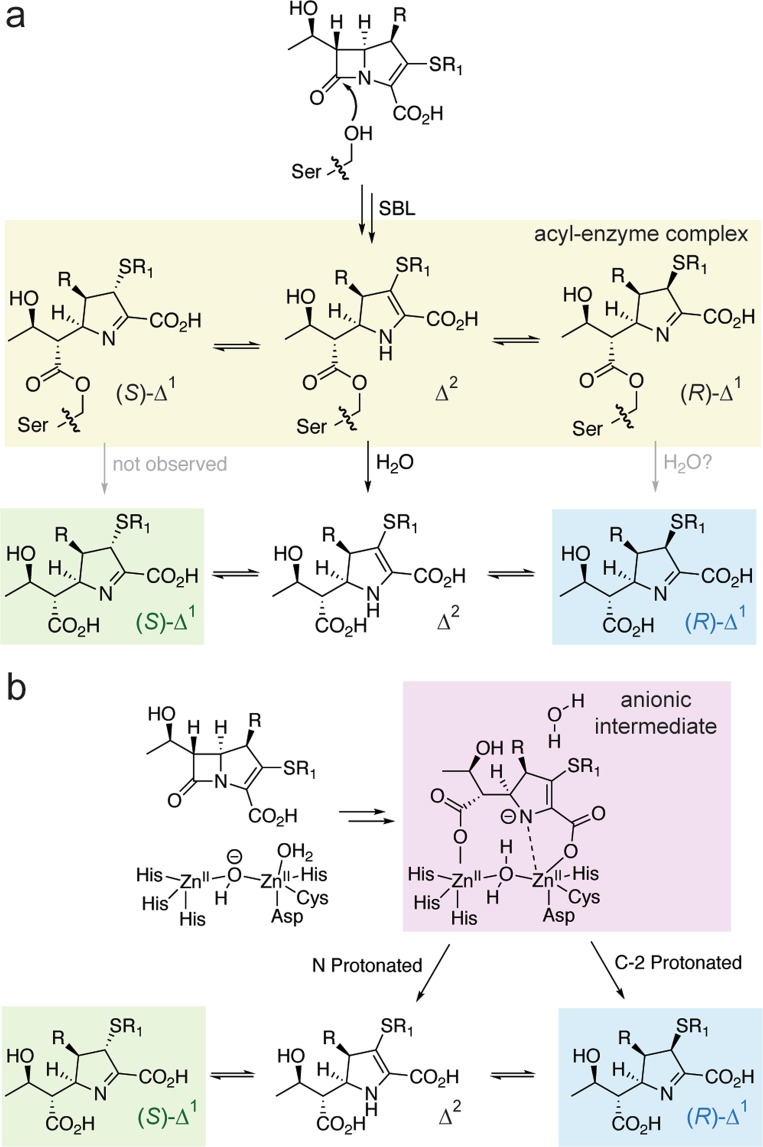
Figure 4.
Mechanistic proposal for the stereoselectivity of carbapenem degradation by serine β-lactamases and metallo-β-lactamases. (a) Scheme showing the proposed outline mechanism of carbapenem degradation by SBLs. Based on our data and previous studies12,14, the Δ2 enamine form of the carbapenem-derived acyl-enzyme complex is likely hydrolysed more rapidly than the Δ1 forms. The Δ2 product, upon release from the enzyme, may then rapidly tautomerise to form the (R)-Δ1 product. Over time, the mixture undergoes further non-enzymatic epimerisation to reach equilibrium, yielding a mixture of the (S)-Δ1 and (R)-Δ1 products. Our NMR data, considered alone, cannot exclude the possibility that the (R)-Δ1 product is at least partially formed enzymatically (though we do not have direct evidence for this). Note that while lactone formation by class D SBLs is not represented in this scheme, its stereoselectivity is expected to be analogous (Supplementary Fig. S12). While the lactone product may then reacylate the enzyme, it is currently unclear which form(s) reacts with the enzyme most efficiently. (b) Scheme showing the proposed outline mechanism of carbapenem hydrolysis by a dinuclear MBL. Following the formation of an anionic intermediate15, protonation either occurs on the nitrogen (forming the Δ2 hydrolysis product) or on C-2 [forming the (R)-Δ1 hydrolysis product]. Note that our data indicates that NMR cannot differentiate between these two possibilities.