Abstract
Leukemia inhibitory factor (LIF) contributes to the neuroprotection by neural stem cells (NSCs) after ischemic stroke. Our aim was to explore whether LIF-transfected NSCs (LIF-NSCs) can ameliorate brain injury and promote neuroprotection in a rat model of cerebral ischemia. To accomplish this goal, we transfected NSCs with a lentivirus carrying the LIF gene to stably overexpress LIF. The LIF-NSCs reduced caspase 3 activation under conditions of oxygen-glucose deprivation in vitro. Transient cerebral ischemia was induced in rats by 2 h of middle cerebral artery occlusion (MCAo), and LIF-NSCs were intravenously injected at 6 h post-ischemia. LIF-NSC treatment reduced the infarction volume and improved neurological recovery. Moreover, LIF-NSCs improved glial cell regeneration and ameliorated white matter injury in the MCAo rats. The NSCs acted as carriers and increased the expression of LIF in the lesions to protect against cerebral infarction, suggesting that LIF-NSCs could be a potential treatment for cerebral infarction.
Keywords: Leukemia inhibitory factor, Neural stem cells, Cerebral ischemia, Infarction volume, Neurological recovery
Introduction
Acute ischemic stroke is a common and frequently-occurring disease that seriously threatens human health [1]. The main pathogenesis of acute ischemic stroke is thromboembolic occlusion of the cerebral artery [2]. The main purposes of cerebral ischemia treatment are to minimize the cerebral infarction area and improve neurological recovery. Previous studies have shown that neural stem cells (NSCs) can generate new neurons and affect neurological recovery [3–5]. The transplantation of NSCs is a promising therapy to improve stroke recovery. However, the low number and survival rate of NSCs after transplantation limit their potential applications in the treatment of stroke [6, 7].
NSCs serve as a gene therapy vector, and gene modification of NSCs by transfection with neurotrophic factors or other effector genes can enhance the secretion of neurotrophic factors and improve the proliferation, survival, and differentiation of endogenous NSCs [8, 9]. Previous studies have indicated that leukemia inhibitory factor (LIF) can significantly enhance the proliferation, survival, and differentiation of NSCs, reduce the oxidative stress and apoptosis of neural precursor cells and neurons, and inhibit inflammatory responses [10–12]. LIF also stimulates the production and survival of neurons and neural support cells (glial cells) [13, 14]. In addition, neuronal damage is accompanied by a transient and rapid increase in LIF expression, suggesting that the action of LIF may be more important in injury responses than in development [15–17]. LIF reduces inflammation and prevents necrosis and apoptosis in NSCs [18, 19] and promotes the migration and differentiation of NSCs, which promotes regeneration in the brain [10]. Thus, we suspected that LIF-transfected NSCs (LIF-NSCs) could be used to treat stroke. Therefore, we isolated NSCs from the mouse brain and cultured and transfected them with a lentivirus encoding the LIF gene in vitro. Then, we investigated the therapeutic efficacy and safety of LIF-NSCs using a transient middle cerebral artery occlusion (MCAo) rat model.
Materials and Methods
Lentivirus Packaging and Characterization of LIF-NSCs
The open reading frame cDNA of LIF was synthesized (Sangon Biotech, Shanghai, China) and inserted into multiple cloning sites of the lentiviral vector pCDH-CMV-MCS-EF1-copGFP (System Biosciences, Mountain View, CA). The constructed vector was verified by sequencing (Sangon Biotech). A lentiviral vector without the LIF sequence was used as a control.
Primary NSCs were derived from the cortex of embryonic mouse brains. The LIF-overexpressing and control viruses were packaged in a lentivirus, and the titer was concentrated to 2 × 108 IU/ml as previously described [20]. Single-cell suspensions of 50,000 NSCs were infected with the virus and termed LIF-NSCs and GFP-NSCs. The NSCs were cultured in proliferation medium (Dulbecco’s modified Eagle’s medium (DMEM), Gibco, Grand Island, NY) supplemented with 2% B-27, 20 ng/ml basic fibroblast growth factor, 20 ng/ml epidermal growth factor (PeproTech, Rocky Hill, NJ, USA), 1% L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 0.1% 2-mercaptoethanol, and 1% penicillin/streptomycin (Gibco) for 3 days at 37°C with 5% CO2. To test the LIF-secreting capacity of the NSCs, the supernatant was collected for LIF detection. To test the LIF-secreting ability of the cells derived from the NSCs, the LIF-NSCs were cultured in differentiation medium (DMEM supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 0.1% 2-mercaptoethanol, and 1% penicillin/streptomycin) for 5 days and then maintained with fresh differentiation medium for another 3 days. The supernatant was collected for LIF detection, and the concentration of LIF was determined using an enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, CA). In vivo, 0.5 g of the brain on the side of the lesion was fragmented with a tissue fragmentation instrument (Qiagen, Hilden, Germany). After centrifugation, the supernatant was collected for LIF detection.
Oxygen-Glucose Deprivation (OGD)
The LIF-NSCs, GFP-NSCs, and NSCs were cultured in 60-mm dishes (Corning, NY, USA) for 3 days; then the cells were digested and seeded on polylysine-pretreated coverslips in 24-well plates as single-cell suspensions of 50,000 cells. The cells were placed in an airtight hypoxic chamber with hypoxic gas (1% O2, 5% CO2, and 94% N2; Thermo, Waltham, MA) and left overnight. Then, the cells were maintained for 2 h under normoxia at 37°C in their respective media. The supernatant was collected from each well, and the LIF assay was performed. The glass coverslips were fixed for 10 min in 4% paraformaldehyde (PFA) for the subsequent immunocytochemical assays.
Immunocytochemistry
Single-cell suspensions of 50,000 cells (LIF-NSCs, GFP-NSCs, and NSCs) were plated on polylysine-pretreated coverslips in a 24-well plates (Corning) and grown at 37°C overnight. The cells were washed gently with phosphate-buffered saline (PBS), fixed with 4% PFA, and blocked with 5% bovine serum albumin (BSA). The cells were incubated with rabbit polyclonal anti-sex-determining region Y-box 2 (SOX2) (1:200, Abcam, Cambridge, UK), rabbit goat polyclonal anti-glial fibrillary acidic protein (GFAP) (1:1000, Abcam), monoclonal anti-Ki67 (1:200, Abcam), and caspase 3 (1:200, Abcam) primary antibodies in 5% BSA at 4°C overnight, followed by incubation with an Alexa Fluor 594-conjugated secondary antibody (1:1000, Invitrogen). Subsequently, the coverslips were washed with PBS and mounted in Fluoroshield mounting medium with 4’, 6-diamidino-2-phenylindole (DAPI; Abcam). The coverslips were finally visualized under a fluorescence microscope (Leica DM6000B, Wetzlar, Germany).
Animal Model of Ischemia and NSC Transplantation
All animal experiments were approved by the Tianjin Medical University Animal Care and Use Committee and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing (280–300) g were maintained in a suitable air-filtered environment and fed normally. The rats were anesthetized with 10% chloral hydrate (3 mL/kg intraperitoneally) and the rectal temperature was maintained at (37 ± 1.0) °C during anesthesia. MCAo was performed as previously described [21]. The rats were subjected to transient (2 h) MCAo via right intraluminal vascular occlusion. The inclusion criteria included visible vessel occlusion on magnetic resonance angiography (MRA) images, recanalization after withdrawal of the occluder, and no intracranial hemorrhage on T2 images 2 h after MCAo. The rats were randomly assigned to three groups: (1) PBS as a control (n = 10), (2) GFP-NSCs (5×106, n = 10), and (3) LIF-NSCs (n = 10) via tail-vein injections starting 6 h post-MCAo. The neurorestorative effects of the LIF-NSCs were investigated 3 and 28 days after stroke in the rats. An investigator blinded to the experimental groups performed a functional evaluation of the modified neurological severity scores (mNSS) before and 1, 2, 3, 7, 14, 21, and 28 days after MCAo. The mNSS is a composite of motor, sensory, balance, and reflex tests and is graded on a scale from 0 to 18 (normal score 0; maximal deficit score 18). One point is awarded for the inability to perform a test or the lack of a tested reflex; thus, higher scores indicate more severe injuries [22].
Magnetic Resonance Imaging (MRI) Measurements
The MRI scans were performed on a 7 T horizontal magnet (ClinScan, Bruker, Karlsruhe, Germany). During the MRI measurements, anesthesia was maintained using a gas mixture of N2O (70%), O2 (30%), and isoflurane (1.0%–1.50%), and the rectal temperature was maintained at (37 ± 1.0) °C using a controlled water bath. Diffusion-weighted imaging (DWI), T2 imaging, diffusion tensor imaging (DTI), or MRA scans were performed 2 h and 1 and 3 days after MCAo. The MRA data were acquired with the following parameters: repetition time (TR)/echo time (TE) = 18/4.1 ms, flip angle = 8°, and spatial resolution = 50 × 50 × 150 μm. The DTI images were acquired from multi-shot spin-echo echo-planar images with 30 diffusion-encoding directions (TR/TE = 3000/35 ms, average = 2, matrix size = 128 × 128 pixels, field of view = 40 × 40 mm2, and single slices with slice thickness = 2.0 mm). The fractional anisotropy (FA) values within the cortex, the basal ganglia (BG), and the corpus callosum (CC) were measured from the right (ischemic) and left (contralateral) hemispheres. The apparent diffusion coefficient (ADC) of the DWI (day 1) and T2 (day 3) images and the FA of the DTI (day 28) images were used to calculate the lesion volume. The rats were sacrificed 28 days after MRI scanning for immunostaining quantification.
Immunohistochemistry
Frozen sections (8 μm thick) through the lesion site were selected. The frozen sections were permeabilized with cold acetone and then blocked (5% BSA). The frozen sections were immunostained with the following primary antibodies at 4°C overnight: rabbit polyclonal anti-LIF (1:200, Abcam), goat polyclonal anti-GFAP (1:1000, Abcam), and mouse anti-myelin basic protein (MBP) (1:200, Millipore, Burlington, USA); subsequently, the sections were incubated with Alexa Fluor 594-conjugated anti-rabbit, anti-goat IgG, or anti-mouse secondary antibodies (1:1000, Invitrogen). The tissue sections were visualized under a Leica DM 6000B microscope. To quantify the number of GFAP-positive cells, four fields of the lesion were acquired. The GFAP-positive cells were counted using image analysis software (ImageJ 1.51j8; National Institutes of Health, Bethesda, MD). The intensities of the MBP immunofluorescence were also analyzed using image analysis software. The data are expressed as percentages of the contralateral fluorescence intensities. An investigator blinded to the experimental groups performed the pathology staining and analysis.
Statistical Analyses
The data are presented as the mean ± SEM. The normally distributed data were analyzed by one-way analysis of variance (ANOVA) and a post hoc test to compare three groups, and a two-tailed unpaired t-test was used to compare two groups. Data with a nonparametric distribution were analyzed with the Mann-Whitney rank sum test. A P-value < 0.05 was considered significant. SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for the analysis.
Results
LIF-NSCs Stably Secreted LIF and Enhanced NSC Proliferation
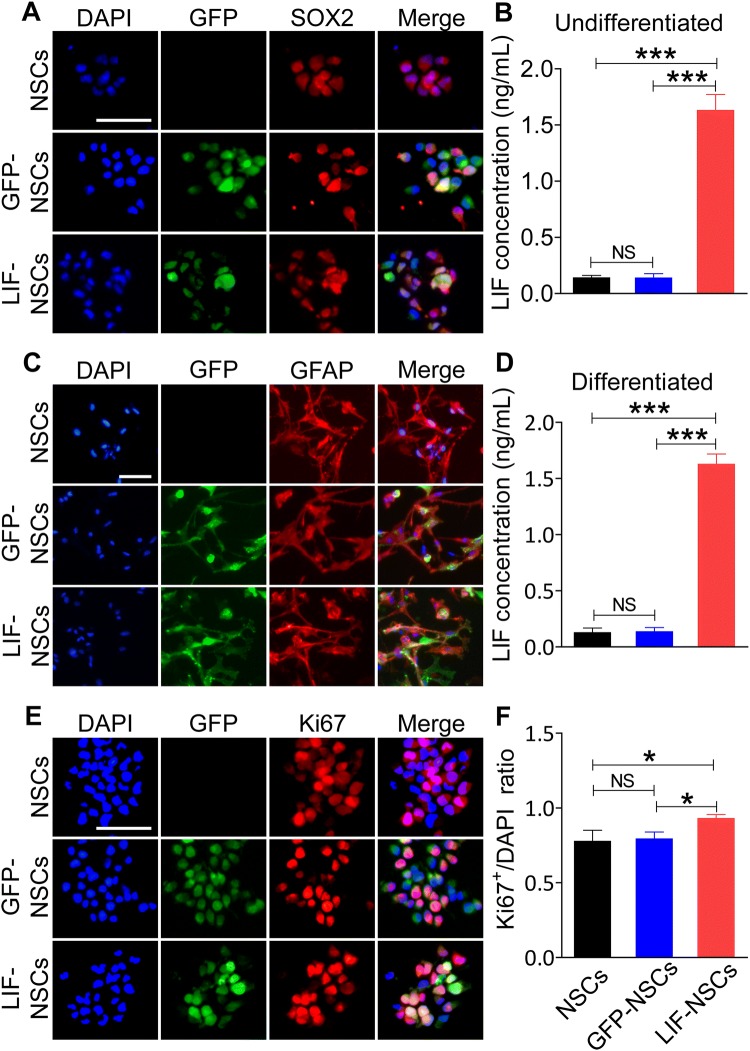
Individual NSCs were transfected with LIF-GFP-overexpressing lentiviruses (LIF-NSCs) or GFP-overexpressing lentiviruses as a control (GFP-NSCs). Both LIF-NSCs and GFP-NSCs expressed GFP (Fig. 1A); however, only the LIF-NSCs secreted high levels of LIF (Fig. 1B). In addition, the GFP-NSCs and LIF-NSCs mostly differentiated into astrocytes in differentiation medium with 10% FBS (Fig. 1C), and the astrocytes derived from the LIF-NSCs continued to secrete high levels of LIF (Fig. 1D). Immunofluorescence staining for Ki67 showed that LIF-NSCs exhibited enhanced proliferation (Fig. 1E–F). These results indicated that LIF-NSCs and their differentiated cells successfully overexpressed LIF and that LIF transfection enhanced NSC proliferation in vitro.
Fig. 1.
LIF-NSCs stably secreted LIF during proliferation and differentiation. A GFP co-localized with SOX2 showed that the control lentivirus vector and vector with the LIF gene were successfully transfected into NSCs. B Concentrations of LIF in the supernatant of LIF-NSCs, GFP-NSCs, and NSCs incubated in proliferation medium for 3 days. C NSCs mostly differentiated into astrocytes in medium with 10% FBS. D NSCs were incubated for 5 days in differentiation medium with 10% FBS, and then the medium was replaced with fresh differentiation medium. After 3 days, the supernatant was collected to measure the concentration of LIF, and the astrocytes derived from the LIF-NSCs also secreted high levels of LIF. E Immunofluorescence staining of Ki67 showing the proliferation ability of LIF-NSCs and GFP-NSCs. F Quantification of the ratio of Ki67+ cells to DAPI-stained cells. Scale bars in (A) (C) and (E), 50 μm. Data are presented as the mean ± SEM; n = 6/group; *P < 0.05, ***P < 0.001.
LIF-NSCs Reduced the Number of Apoptotic Cells Following OGD in Vitro
NSCs, GFP-NSCs, and LIF-NSCs were incubated in proliferation medium for 3 days and then plated on polylysine-pretreated coverslips in a 24-well plates with original medium overnight. Caspase 3 was used as an indicator of apoptotic cells [23, 24]. Compared with the NSC and GFP-NSC groups, under the OGD condition, the LIF-NSC group exhibited a markedly reduced percentage of caspase 3-positive cells (Fig. 2A, B) (P < 0.001). The LIF concentrations in the NSC culture supernatant after OGD were measured by ELISA. The results showed that hypoxia increased the expression of LIF in the NSCs, especially in the LIF-NSCs (Fig. 2C) (P < 0.01).
Fig. 2.
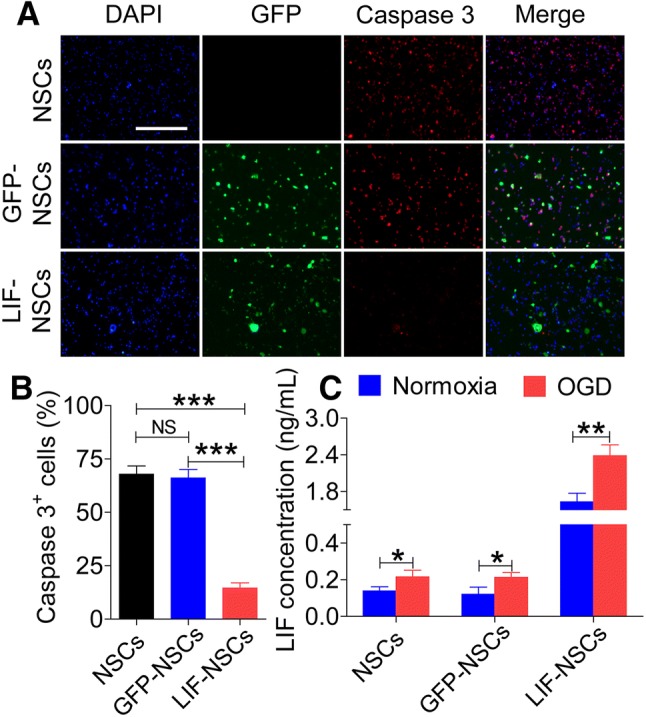
LIF-NSCs reduced the number of apoptotic cells following OGD in vitro. A Immunofluorescence staining of caspase 3 (red) and DAPI (blue). B Quantification of active caspase 3 staining for caspase 3+ cells. C Concentrations of LIF in the supernatant after OGD and under normoxic conditions. Scale bar, 200 μm. Data are presented as the mean ± SEM; n = 6/group; *P < 0.05, **P < 0.01, ***P < 0.001.
LIF-NSC Treatment Decreased the Lesion Volume and White Matter Injury, and Improved the Long-Term Functional Outcome
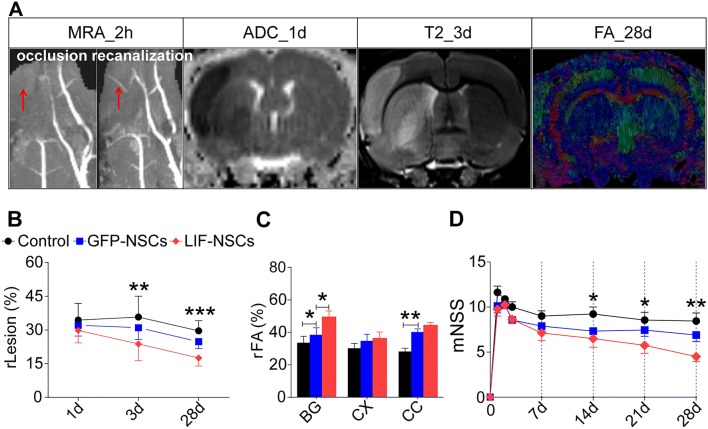
The long-term lesion benefit derived from LIF-NSC treatment initiated 3 days after MCAo was assessed using a battery of MRI sequences. In total, n = 10/group was used with a mortality rate of 20% (within 3 days after MCAo); thus, 8 rats/group survived at the end of the experiments in both the treatment and control groups. The FA, ADC, and T2 images are presented in Fig. 3A. A significantly reduced lesion volume was found in the MCAo rats treated with LIF-NSCs compared with that in the GFP-NSC-treated MCAo rats at day 3 (P < 0.01) and day 28 (P < 0.001), but the GFP-NSC-treated rats did not exhibit a significant reduction in lesion volume compared with the PBS-treated rats (Fig. 3B).
Fig. 3.
Long-term function and MR evolution after LIF-NSC treatment in rats after MCAo. A FA images of DTI and ADC of DWI and T2 images at multiple time points (1, 3, and 28 days after ischemia) from a single representative animal as shown by MRA. B Changes in lesion volume over time. ADC of DWI (day 1) and T2 (day 3) and FA images (day 28) were used to calculate the lesion volume; relative lesion (rLesion) = lesion volume/right hemisphere volume. C Differences in FA values at day 28; FA values in the cortex, basal ganglia (BG), and corpus callosum (CC) were measured in the right (ischemic) and left (contralateral) hemispheres; relative FA (rFA) = mean FA value of lesion/mean FA value of corresponding left hemisphere. D Change in the modified neurological severity score (mNSS) over time. Data are presented as the mean ± SEM; n = 8 /group; *P < 0.05, **P < 0.01, ***P < 0.001.
During the hyperacute phase, the FA values often show contradictory results due to differences in edema progression. However, during the chronic phase, the FA values are stable and correlated with tissue, motor, and neurological recovery. Therefore, in this study, we only measured the FA value on day 28 after ischemia. In the cortex, BG and CC, FA failed to normalize but remained low on day 28 after MCAo, suggesting a breakdown of the cell membrane and disruption of the cytoarchitecture in the white matter. However, after LIF-NSC or GFP-NSC treatment, the FA values in the BG and CC were higher than those in the control group, and the FA value in the BG after LIF-NSC treatment was significantly higher than that after GFP-NSC treatment (Fig. 3C).
The long-term functional benefit derived from LIF-NSC treatment began 14 days after MCAo as assessed using the mNSS test. However, GFP-NSC treatment did not decrease the functional disability compared with controls (Fig. 3D).
LIF-NSC Treatment Increased the Expression of LIF and Improved Glial Cell Regeneration in vivo
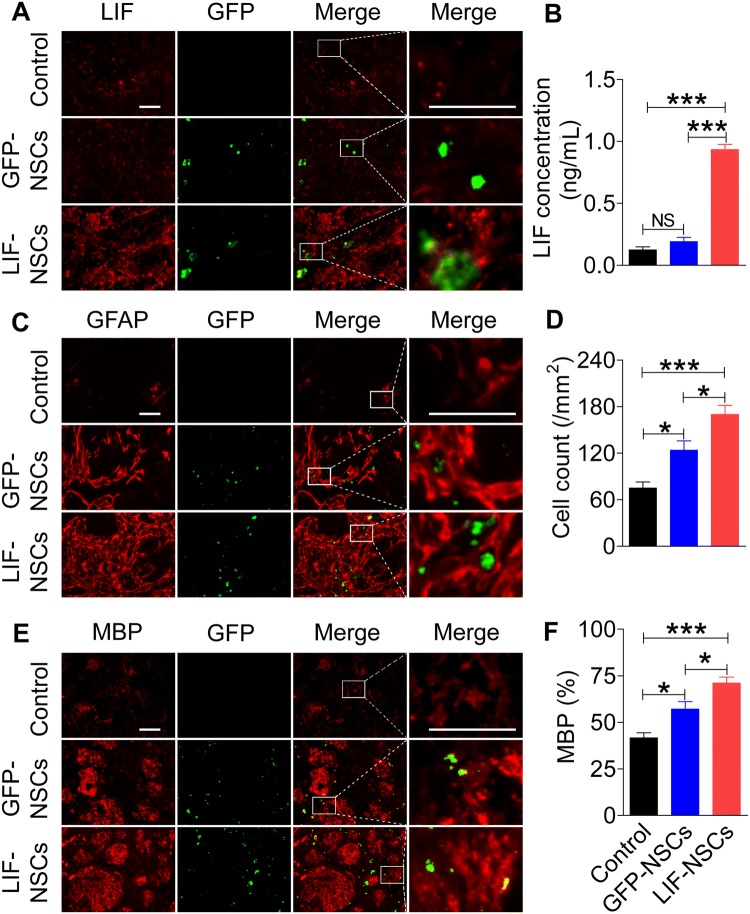
Both the LIF-NSC- and GFP-NSC-treated groups showed high GFP expression in the ipsilateral striatum (Fig. 4A), where immunohistochemistry revealed that only LIF-NSCs secreted higher levels of LIF (Fig. 4A). The LIF protein levels in the ipsilateral striatum were also detected by ELISA. Compared with PBS and GFP-NSC treatment, LIF-NSC treatment increased the expression of LIF in the brain (Fig. 4B). LIF-NSC treatment also promoted the formation of glia. At 28 days after MCAo, the LIF-NSC-treated rats had significantly more astrocytes at the lesion site than the control and GFP-NSC-treated rats (Fig. 4C–D). MBP staining to evaluate the effects of LIF-NSC treatment on white-matter remodeling revealed higher fluorescence intensity in the white-matter bundles of the LIF-NSC group than in the control group (Fig. 4E–F).
Fig. 4.
LIF-NSC treatment increased LIF expression in the brain and promoted glial cell regeneration. A LIF-NSC- and GFP-NSC-treated groups showed a high expression of GFP in the ipsilateral striatum, and only the LIF-NSCs secreted higher levels of LIF. B Concentrations of LIF using ELISA; LIF-NSC treatment increased the expression of LIF compared with that in the control and GFP-NSC-treated MCAo rats. C Immunofluorescence staining of GFAP in the different groups 28 days after MCAo. D Quantification of GFAP+ cells showing that the LIF-NSC treatment group had significantly more astrocytes than the control and GFP-NSC treatment groups of MCAo rats. E Immunofluorescence staining of MBP. F Quantification of MBP immunofluorescence expressed as the percentage of contralateral fluorescence intensity. The LIF-NSC treatment group had higher fluorescence intensity in the white matter bundles than the control and GFP-NSC treatment groups. Scale bars, 50 μm in A, C, and E. Data are presented as the mean ± SEM; n = 8 /group; *P < 0.05, ***P < 0.001.
Discussion
Here, we showed that overexpressing LIF enhances the therapeutic action of NSCs in rats after transient focal ischemia. This effect was achieved by intravenously transplanting NSCs modified with LIF (LIF-NSCs) into rats in a stroke model. LIF transfection enhanced the proliferation of NSCs, and both LIF-NSCs and their differentiated astrocytes successfully secreted LIF at a relatively high level. We found that LIF-NSC treatment improved functional recovery and decreased the lesion volume based on MRI measurements in MCAo rats. Moreover, based on pathological assessment, LIF-NSC treatment also promoted white-matter remodeling and increased glial cell regeneration.
In this study, the genetic modification of NSCs with LIF enhanced their beneficial effects of reducing the infarction volume and improving neurological recovery after cerebral ischemia, especially during the chronic phase. MRI showed clear enhancement in the recovery of FA values in the BG after LIF-NSC treatment, and these values were stable and correlated with tissue, motor, and neurological recovery. In contrast to our study, NSCs derived from human induced pluripotent stem cells survive and differentiate into neurons but do not reduce the infarct volume or improve the behavioral recovery of ischemic rats [25]. This lack of a therapeutic effect may be related to a difference in the LIF concentration in the microenvironment of the focal area after cerebral ischemia [18, 26, 27].
LIF promotes the maintenance of NSCs in vivo and in vitro [28, 29]. Brain injury is a potent inducer of LIF expression, which increases subventricular zone or hippocampal neuronal proliferation possibly by promoting NSC self-renewal, suggesting that LIF may be a key signaling molecule after injury [10, 30]. LIF also stimulates the production and survival of neurons and neural support cells (glial cells) that can affect neuronal connectivity [31]. LIF increases the number of GFAP+ cells and promotes the differentiation of astrocyte progenitor cells into mature GFAP+ astrocytes [14, 32]. Similarly, LIF induces the production, maturation, and survival of oligodendrocytes from oligodendrocyte precursor cells [13]. LIF stimulates the proliferation of these cells and enhances oligodendrocyte remyelination in the hippocampus after demyelination [33]. LIF also stimulates the production and survival of neurons [34]. Specifically, in this study LIF-NSCs increased the content of LIF; regardless of whether most of the LIF-NSCs differentiated into astrocytes in the damaged areas, they still secreted LIF. The elevated concentration of LIF decreased the ischemic damage following LIF-NSC treatment, suggesting that the upregulation of LIF was responsible for the enhanced protective effects against stroke.
We transplanted heterologous NSCs into the rat tail vein. The intravenous administration of NSCs is minimally invasive and an effective and safe procedure for stroke treatment. The genetic modification of NSCs can enhance the survival, migration, proliferation, and neurotrophic factor secretion of NSCs [8, 9]. Compared with non-genetically modified NSCs, similar numbers of engrafted LIF-NSCs were detected in the ischemic hemisphere after MCAo. These results indicated that engrafted LIF-NSCs rarely improved the injured brain by increasing the survival and migration of grafted NSCs to the injured site after ischemic stroke. LIF was highly expressed by LIF-NSCs and astrocytes derived from them under normal physiological conditions, which may strongly promote endogenous NSC proliferation. The LIF-NSCs also exhibited upregulated LIF expression under conditions of hypoxia, thereby decreasing endogenous NSC apoptosis. However, our study also has limitations. The LIF-transfected NSCs had a low survival rate in the brains of MCAo rats. Further studies are needed to explore new techniques to improve the quantity of exogenous LIF in the brain. For example, NSC survival after the intracerebral transplantation of NSCs into the stroke brain has been enhanced by the use of biomaterial scaffolding and minocycline preconditioning [35]. In addition, other types of cells transfected with LIF (e.g., mesenchymal stem cells) may be substituted.
In summary, LIF-NSCs reduced the infarct volume and promoted functional repair in MCAo rats, indicating that LIF-NSCs may serve as a novel treatment for cerebral infarction. Furthermore, our results suggested that LIF from engrafted LIL-NSCs probably promotes endogenous cell proliferation and decreases apoptosis, which may contribute to recovery from neurological disability and tissue injury after stroke. However, the fate of NSCs in the brain remains to be shown, and the conclusion that LIF is neuroprotective in cerebral ischemia remains to be confirmed.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81571596, 81601044, and 81771279), the National Basic Research Development Program of China (2017YFC1701300), and Fundamental Research Funds for the Central Universities, China (GK201701009).
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Yi Lin, Email: linyi7811@163.com.
Yaping Yan, Email: yaping.yan@snnu.edu.cn.
References
- 1.Meairs S, Wahlgren N, Dirnagl U, Lindvall O, Rothwell P, Baron JC, et al. Stroke research priorities for the next decade–A representative view of the European scientific community. Cerebrovasc Dis. 2006;22:75–82. doi: 10.1159/000093098. [DOI] [PubMed] [Google Scholar]
- 2.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke: Neurological diseases. Nat Rev Neurosci. 2003;4:399–414. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 3.Chu K, Kim M, Park K-I, Jeong S-W, Park H-K, Jung K-H, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum: Transplantation of neural stem cells from human fetal brain. Eur J Neurosci. 2007;26:605–614. doi: 10.1111/j.1460-9568.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 7.Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis. 2013;49:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenny B, Kanemitsu M, Tsupykov O, Potter G, Salmon P, Zgraggen E, et al. Fibroblast growth factor-2 overexpression in transplanted neural progenitors promotes perivascular cluster formation with a neurogenic potential. Stem Cells. 2009;27:1309–1317. doi: 10.1002/stem.46. [DOI] [PubMed] [Google Scholar]
- 9.Zhu W, Mao Y, Zhao Y, Zhou L-F, Wang Y, Zhu J-H, et al. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery. 2005;57:325–333. doi: 10.1227/01.NEU.0000166682.50272.BC. [DOI] [PubMed] [Google Scholar]
- 10.Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26:12089–12099. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26:533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiura S, Lahav R, Han J, Kou S-Y, Banner LR, De Pablo F, et al. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro: LIF in neural injury. Eur J Neurosci. 2000;12:457–466. doi: 10.1046/j.1460-9568.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 13.Rowe DD, Collier LA, Seifert HA, Chapman CB, Leonardo CC, Willing AE, et al. Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. Eur J Neurosci. 2014;40:3111–3119. doi: 10.1111/ejn.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida T, Satoh M, Nakagaito Y, Kuno H, Takeuchi M. Cytokines affecting survival and differentiation of an astrocyte progenitor cell line. Brain Res Dev Brain Res. 1993;76:147–150. doi: 10.1016/0165-3806(93)90132-T. [DOI] [PubMed] [Google Scholar]
- 15.Banner LR, Moayeri NN, Patterson PH. Leukemia inhibitory factor is expressed in astrocytes following cortical brain injury. Exp Neurol. 1997;147:1–9. doi: 10.1006/exnr.1997.6536. [DOI] [PubMed] [Google Scholar]
- 16.Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, et al. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe SM. LIF in the regulation of T-cell fate and as a potential therapeutic. Genes Immun. 2011;12:157–168. doi: 10.1038/gene.2011.9. [DOI] [PubMed] [Google Scholar]
- 19.Weber MA, Schnyder-Candrian S, Schnyder B, Quesniaux V, Poli V, Stewart CL, et al. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab Invest. 2005;85:276–284. doi: 10.1038/labinvest.3700216. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y, Ding X, Li K, Ciric B, Wu S, Xu H, et al. CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes. Mol Ther. 2012;20:1338–1348. doi: 10.1038/mt.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12:621–628. doi: 10.1038/jcbfm.1992.86. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.STR.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 23.Hall AA, Guyer AG, Leonardo CC, Ajmo CT, Collier LA, Willing AE, et al. Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. J Neurosci Res. 2009;87:333–341. doi: 10.1002/jnr.21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Z, Pan K, Pan J, Peng Q, Wang YJ. The Possibility and molecular mechanisms of cell pyroptosis after cerebral ischemia. Neurosci Bull. 2018;34:1131–1136. doi: 10.1007/s12264-018-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen MB, Yan H, Krishnaney-Davison R, Al Sawaf A, Zhang S-C. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J Stroke Cerebrovasc Dis. 2013;22:304–308. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators: neuroinflammatory mediators in brain ischemia. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 27.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leuko Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ. LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci. 2004;27:255–266. doi: 10.1016/j.mcn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr BJ, Patterson PH. Potent pro-inflammatory actions of leukemia inhibitory factor in the spinal cord of the adult mouse. Exp Neurol. 2004;188:391–407. doi: 10.1016/j.expneurol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Zhou LJ, Liu XG. Glial Activation, A common mechanism underlying spinal synaptic plasticity? Neurosci Bull. 2017;33:121–123. doi: 10.1007/s12264-016-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards LJ, Kilpatrick TJ, Dutton R, Tan S-S, Gearing DP, Bartlett PF, et al. Leukaemia inhibitory factor or related factors promote the differentiation of neuronal and astrocytic precursors within the developing murine spinal cord. Eur J Neurosci. 1996;8:291–299. doi: 10.1111/j.1460-9568.1996.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 33.Deverman BE, Patterson PH. Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J Neurosci. 2012;32:2100–2109. doi: 10.1523/JNEUROSCI.3803-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis SM, Collier LA, Leonardo CC, Seifert HA, Ajmo CT, Pennypacker KR. Leukemia inhibitory factor protects neurons from ischemic damage via upregulation of superoxide dismutase 3. Mol Neurobiol. 2017;54:608–622. doi: 10.1007/s12035-015-9587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]