Abstract
Background.
Introduction of an organic diet can significantly reduce exposure to some classes of pesticides in children and adults, but no long-term trials have been conducted.
Objectives.
To assess the effect of a long-term (24-week) organic produce intervention on pesticide exposure among pregnant women.
Methods.
We recruited 20 women from the Idaho Women, Infants, and Children (WIC) program during their first trimester of pregnancy. Eligible women were nonsmokers aged 18-35 years who reported eating exclusively conventionally grown food. We randomly assigned participants to receive weekly deliveries of either organic or conventional fruits and vegetables throughout their second or third trimesters and collected weekly spot urine samples. Urine samples, which were pooled to represent monthly exposures, were analyzed for biomarkers of organophosphate (OP) and pyrethroid insecticides.
Results.
Food diary data demonstrated that 66% of all servings of fruits and vegetables consumed by participants in the “organic produce” group were organic, compared to <3% in the “conventional produce” group. We collected an average of 23 spot samples per participant (461 samples total), which were combined to yield 116 monthly composites. 3-phenoxybenzoic acid (3-PBA, a non-specific biomarker of several pyrethroids) was detected in 75% of the composite samples, and 3-PBA concentrations were significantly higher in samples collected from women in the conventional produce group compared to the organic produce group (0.95 vs 0.27 μg/L, p=0.03). Another pyrethroid biomarker, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid, was detected more frequently in women in the conventional compared to the organic produce groups (16% vs 4%, p=0.05). In contrast, we observed no statistically significant differences in detection frequency or concentrations for any of the four biomarkers of OP exposure quantified in this trial.
Discussion.
To our knowledge, this is the first long-term organic diet intervention study, and the first to include pregnant women. These results suggest that addition of organic produce to an individual’s diet, as compared to conventional produce, significantly reduces exposure to pyrethroid insecticides.
1. Introduction
A growing number of prospective birth cohort studies have investigated the relationship between in utero exposure to organophosphate (OP) and pyrethroid pesticides and neurodevelopmental outcomes in children. Many, though not all, of these studies have concluded that higher prenatal exposure to these insecticides is associated with poorer neurological and cognitive development in children (Bouchard et al. 2011; Cartier et al. 2016; Engel et al. 2011; Engel et al. 2016; Eskenazi et al. 2014; Furlong et al. 2017; Marks et al. 2010; Sagiv et al. 2018; Stein et al. 2016; Viel et al. 2015; Viel et al. 2017). Several systematic reviews have critically evaluated the available body of work regarding the neurodevelopmental impacts of prenatal pesticide exposure, and while these reviews have primarily focused on OPs, the collective evidence supports the hypothesis that such exposure induces neurotoxic effects (Muñoz-Quezada et al. 2013; González-Alzaga et al. 2014; Hernández et al. 2016). This evidence has motivated calls to reduce exposure to neurotoxic chemicals, like OP and pyrethroid pesticides, in vulnerable populations (Bennett et al. 2016; Hertz-Picciotto et al. 2018).
OP pesticides were broadly used in the USA in residential settings until the mid-2000s when residential uses of chlorpyrifos and diazinon were phased out (US EPA 2000, 2001); residential use of pyrethroids remains common and represents a potential exposure pathway (Palmquist et al. 2012). Diet is also a potentially important source of exposure to both OP and pyrethroid pesticides (Melnyk et al. 2014; Nougadère et al. 2012; Oates and Cohen 2011), as OP and pyrethroid pesticides are widely used in agriculture (Roberts and Reigart 2013). However, usage patterns of these pesticides are changing. While OP pesticides have been the dominant class of insecticides used in American agriculture during the past two decades, OP use has declined more than 70% since 2000, from an estimated 70 million pounds to 20 million pounds in 2012 (EPA 2017). As a percentage of total insecticidal use, OP usage has dropped from 71% in 2000 to 30% in 2012, and this drop reflects a shift to usage of other classes of insecticides, including pyrethroids (EPA 2017).
Use of these synthetic pesticides is prohibited in the production of food certified as organic, and food monitoring has confirmed that organically grown food typically contains lower pesticide residues than food that is conventionally grown (Baker et al. 2002; USDA 2011). Further, both observational and short-term experimental studies have shown that consumption of an organic diet is associated with a significant reduction in biomarkers of OPs and, to a lesser degree, pyrethroids, when compared to consumption of a conventional diet (Berman et al. 2016; Bradman et al. 2015; Curl et al. 2003; Curl et al. 2015; Göen et al. 2017; Hyland et al. 2019; Lu et al. 2006b; Lu et al. 2008; Lu et al. 2009; Oates et al. 2014).
However, despite evidence suggesting that low-dose In utero exposure to OP and pyrethroid pesticides is associated with negative health effects, and compelling data showing an organic diet to be an effective means of lowering exposures to these compounds, it remains difficult to draw conclusions about any potential health benefits of an organic diet. This is because most existing birth cohorts were not focused on diet, and were established either in primarily agricultural regions (Eskenazi et al. 1999; Petit et al. 2010) or in urban areas where residential pesticide use was common (Whyatt et al. 2004; Wolff et al. 2007). Because diet was not necessarily a primary source of pesticide exposure in these cohorts, it remains unknown whether pesticide exposure from consumption of a conventional diet is sufficiently high to lead to the adverse health outcomes observed. Further, in the HOME Study where dietary intake was the primary source of OP pesticides, the investigators found adverse effects of prenatal OP exposure on birth weight and gestational age, but not neurodevelopmental outcomes (Donauer et al. 2016; Rauch et al. 2012).
We propose that observational studies of the relationship between organic food consumption and health will always be limited by the potential for uncontrolled confounding. In both the United States and Europe, individuals who consume organic food generally tend to report other factors associated with better health, including higher levels of educational attainment and higher income (Curl et al. 2013; Dettmann and Dimitri 2009; Govindasamy and Italia 1999; Petersen et al. 2013; Simões-Wüst et al. 2017; Smith et al. 2009; Williams and Hammitt 2001; Zhang et al. 2008). Organic food consumption is also associated with greater total fruit and vegetable intake (Curl et al. 2015; Hu et al. 2016; Petersen et al. 2013), which itself confers health benefits. These associations hinder investigations of the relationship between organic food consumption and health effects using an observational study design.
An experimental design would represent a compelling way to evaluate this relationship but to date, no organic diet trials have been tested in pregnant women. In addition, all organic diet trials to date have been brief, typically lasting between four days and two weeks. Finally, existing organic diet trials have included a completely organic diet, which may not represent realistic eating habits of most consumers of organic food. The primary objective of this study was to test the efficacy of a long-term (24-week) randomized organic diet trial on pesticide exposure in pregnant women. This trial involved provision of either organic or conventional fruits and vegetables as a supplement to the participants’ regular diets.
This study focused on supplementing diets with fruits and vegetables for several reasons. First, fruits and vegetables have been the top selling category of organically grown food since the organic food industry began retailing products in the 1990s, and they continue to outsell other organic food categories (USDA 2017). Second, insecticides such as OPs and pyrethroids are more commonly used in crop production than in livestock or dairy, and residues of these pesticides are more commonly found on produce items than animal-based products such as milk (USDA 2017). Finally, most organic consumers report that they “sometimes” consume organic food, while fewer than 5% report that they “often or always” do (Curl et al. 2015). Therefore, to better represent the actual consumption habits of most organic consumers and to focus on food items most likely to influence exposure to OP and pyrethroid pesticides, we elected to supplement participants’ diets with either organic or conventional produce, rather than fully replacing conventional diets with organic food. We hypothesized that those receiving organic fruits and vegetables would have lower exposures to OP and pyrethroid insecticides than those receiving conventional produce.
2. Materials and Methods
2.1. Study Participants.
Participants were recruited between June and December of 2016 from Women, Infants and Children (WIC) clinics in urban and suburban regions of the Treasure Valley of Idaho, including the cities of Boise, Meridian, and Garden City. Treasure Valley WIC clinics serve over 500 pregnant women per month who meet income guidelines of 185% poverty level or lower. Clinic staff and counselors used an informational script to identify and screen interested participants for eligibility. Criteria for eligibility included: pregnancy less than 16-weeks gestation; 18 to 35 years of age; consumer of a fully conventional (non-organic) diet; non-smoker; no report of alcohol consumption during this pregnancy; no report of occupational exposure to pesticides; and no history of high-risk pregnancy or gestational diabetes. WIC counselors recited the prepared script and obtained permission from interested women to submit their contact information to the researcher staff. Research staff then contacted potential participants via telephone to confirm interest and eligibility. An initial home visit was scheduled, during which researcher staff presented the study purpose and protocols, obtained written consent, and administered a pesticide exposure questionnaire. Produce delivery and urine sample collection began in July of 2016 and continued through May 2017. All procedures were reviewed and approved by the Boise State University Human Subjects Institutional Review Board. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
2.2. Pesticide Exposure Questionnaire.
All participants were interviewed in person using a standardized questionnaire to collect demographic data and identify any non-dietary sources of pesticide exposure. Demographic data included age, family income, highest level of educational attainment, and race/ethnicity. Participants were then asked a series of questions to evaluate use of pesticides to control any of the following: insects inside or outside of their residences; weeds; plant diseases; snails and slugs; and any larger pests (e.g., rats, mice). Participants were also asked about pet ownership and about any pesticides used to control fleas or ticks on those pets. Finally, researchers recorded information on any pesticides from the containers that the residents reported using.
2.3. Trial Design and Diet Protocol.
All study participants had the opportunity to receive as many as 24 consecutive weekly deliveries of $20-worth of fresh fruits and vegetables, for a total of $480-worth of free produce throughout their second and third pregnancy trimesters. We designed a parallel trial with a 1:1 allocation ratio, in which participants were randomly assigned to either the “organic” or the “conventional” group, with those in the organic group receiving organic produce and those in the conventional group receiving conventional produce. Prior to participant enrollment, a research staff member used a computer program to generate a sequence of random numbers. As participants were recruited to the study, their study ID numbers were matched to this sequence of numbers; participants who were matched with even numbers were assigned to the conventional arm and those matched with odd numbers were assigned to the organic arm. The research staff member who generated the ID numbers and group assignments was involved in participant enrollment activities, but she was not involved in the data analysis. Participants were not informed of their group assignments.
Participants placed individual produce orders each week at least three days prior to their established food delivery date. Orders were made through a password-protected website developed by the research team in collaboration with the Boise State University Office of Information Technology. An automated reminder email was sent to any participant who did not submit an order prior to this date. This “Produce Order Form” contained a list of approximately 25 different common fruits and vegetables (apples, bananas, spinach, tomatoes, carrots, onions, blueberries, etc.), each with an associated price reflecting the average of the price of the organic and conventional versions of the item. Participants indicated the number (in pounds, bunches or pints, as appropriate) of each item they wanted to receive, and the Order Form calculated the total cost, which was capped at $20 per week. All fruits and vegetables listed on the Order Form were available from grocery suppliers in both organic and conventional varieties. Seasonal changes in fruit and vegetable availability occasionally altered the options offered. Participants’ orders were captured in an Order Database, and local grocery suppliers automatically received compiled weekly lists of the orders. These suppliers delivered all produce to the “Clean Kitchen” area of our research laboratory on an established schedule. Research staff ensured that all labels or stickers that might identify the produce as organic or conventional were removed, and packaged each participant’s individual order. A delivery company then distributed the produce to all participants.
Participants were asked to incorporate these produce items into their existing diet, but were not required to only eat produce provided by the study. Participants were not discouraged from eating at restaurants or at other people’s homes, and were not discouraged from eating additional fruits and vegetables that we did not deliver. We also said that they could share the produce we delivered with their families. We allowed this for three reasons. First, because this was a long-term study, we were concerned that participants would not be compliant with a more restrictive diet, particularly during pregnancy. We also did not want to limit their fruit and vegetable intake. Finally, our previous research has shown that most people who report consuming organic food do so “occasionally”; few “always” consume organic food (Curl et al. 2015). In this study, we wanted to evaluate the magnitude of difference in pesticide biomarker concentrations associated with a realistic frequency of organic food consumption that might reasonably mimic the actual habits of most consumers of organic foods.
2.4. Food Records.
In conjunction with Boise State University’s Office of Information Technology, we developed a study-specific phone app to allow participants to track all of the fruits and vegetables they consumed. This app is available for Android or iOS and can be found in most app stores under “CAHL Food Record” (Curl Agricultural Health Lab). All participants with smartphones were asked to download this app and use it to record all fruits and vegetables they consumed throughout the 24-week study period. This password-protected app allowed participants to take a picture of any meal or snack that included a fruit or vegetable. They then indicated the meal type (e.g., breakfast, lunch, dinner or snack), selected the item type from a drop-down menu (e.g., orange, banana), and selected the portion size consumed. Participants were provided a Portion Size Guide to assist in identifying portion sizes.
Participants were then asked whether the item was provided by the study; if the item was not provided by the study, they were asked whether it was organic or conventional. While the participants were blinded to their group assignment, the research staff coding the dietary data were not. Therefore, staff were able to appropriately designate produce reported as “provided by the study” based on group assignment. Produce items that were not provided by the study were coded as “organic” or “conventional” based on the designation provided by the participant. Data were then downloaded into a Food Record Database that tracked the number of servings of each type of fruit and vegetable item consumed by each participant over the course of the study. These totals were calculated separately for servings of organic and conventional food.
Written food diaries were provided to three participants who did not have a smartphone or who did not want to use a phone app. These diaries captured the same information as the phone app but did not allow participants to photograph their meals; written diary data was hand-entered into the same Food Record Database.
2.5. Urine Specimen Collection.
We collected one baseline, pre-intervention spot urine sample and a series of up to 24 weekly spot urine samples during the study period from each participant. Research staff visited each participant’s home every week, provided participants with a pre-labeled 4-oz polypropylene specimen cup, and asked them to provide a urine sample. Participants were asked to collect at least 10 mL of urine. Research staff recorded the date and time of sample receipt on a Chain of Custody form, which was also signed by the participants. Urine cups were placed into resealable plastic bags and transported on ice back to the laboratory at Boise State University, where they were analyzed for specific gravity via refractometry (Atago Urine Specific Gravity Refractometer, PAL 10-S).
Individual weekly urine samples collected during the dietary intervention were pooled to create monthly composites for analysis. Specifically, one mL from each of four separate weekly spot samples was pipetted into a 5-mL cryovial, intended to represent exposure over the course of a given month. All samples were stored at −80 °C within 48 hours of collection; additional aliquots were added in layers to a given cryovial as each month progressed. Samples were shipped on dry ice to the CDC, National Center for Environmental Health in Atlanta, Georgia for analysis within nine months of collection. Samples were submitted for analyses in one of two shipments, and all samples collected from a given participant were analyzed together within the same analysis batch.
2.6. Quantification of Pesticide Biomarkers in Urine Specimens.
Urine samples were analyzed at the CDC for four specific metabolites of organophosphate insecticides: 3,5,6-trichloro-2-pyridinol (TCPY, a metabolite of chlorpyrifos and chlorpyrifos-methyl), 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMPY, a metabolite of diazinon), para-nitrophenol (PNP, a metabolite of parathion and methyl parathion as well as other chemicals), and malathion dicarboxylic acid (MDA, a metabolite of malathion). Samples were also analyzed for three metabolites of pyrethroid insecticides: 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA, a metabolite of cyfluthrin and flumethrin), 3-phenoxybenzoic acid (3-PBA, a nonspecific metabolite of several pyrethroids including cyhalothrin, cypermethrin, deltamethrin, fenpropathrin, permethrin, and tralomethrin), and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (trans-DCCA, a metabolite of permethrin, cypermethrin, and cyfluthrin).
Target analytes were extracted using semi-automated solid phase extraction, separated using reversed-phase high-performance liquid chromatography, and detected using tandem mass spectrometry with isotope dilution quantitation. Method details and quality control procedures have been previously described (Davis et al. 2013). Limits of detection (LODs) were 0.1 μg/L (TCPY, IMPY, PNP, 4-F-3-PBA, 3-PBA); 0.5 μg/L (MDA); and 0.6 μg/L (trans-DCCA). One sample result for 3-PBA, one sample result for PNP and one sample result for trans-DCCA could not be reported due to an interfering substance during the analysis. The CDC laboratory is certified by the Health Care Financing Administration to comply with the requirements set forth in the Clinical Laboratory Improvement Act of 1988 (CLIA ‘88) and is recertified annually. Therefore, the analytical measurements followed strict CLIA-recommended quality control/quality assurance protocols. For example, in addition to study samples, each analytical run included calibration standards, two high- and two low-concentration quality control (QC) materials (prepared using pooled human urine), and blanks to ensure data accuracy and reliability. The concentrations of the high-concentration QCs and the low-concentration QCs, averaged to obtain one measurement of high-concentration QC and low-concentration QC for each run, were evaluated using standard statistical probability rules (Caudill et al., 2008). If the QC samples failed the statistical evaluation, all of the study samples in the run were re-extracted.
2.7. Data Analysis.
We summarized study participants’ demographic characteristics, and compared them by group assignment. We also evaluated all participants’ other potential sources of pesticide exposure, based on data collected through the Pesticide Exposure Questionnaire. We then evaluated the frequency of detection of each biomarker during the intervention period in composite samples from the organic and conventional groups. We compared the frequency of detection of each biomarker between the organic and conventional groups using the Fisher exact test.
In addition to comparing detection frequencies, we also compared biomarker concentrations in the two groups. Based on previously published results, we anticipated that not all of the biomarkers analyzed in this study would be frequently detected. Therefore, we decided a priori to focus analyses on biomarkers with no more than 70% censored data because at degrees of censoring higher than 70%, no techniques for substituting data provide good estimates of summary statistics, including maximum likelihood estimation (MLE) methods (Antweiler and Taylor 2008). For biomarkers detected in at least 30% of samples, non-detectable concentrations were replaced with a value equal to the LOD divided by the square root of 2 (Hornung and Reed 1990). Prior to all analyses, we employed specific gravity measurements to adjust biomarker concentrations for urine dilution according to:
where CSG is the adjusted result (μg/L), C is the original measured concentration (μg/L), 1.016 is the mean specific gravity measured within the study population, and SG is the specific gravity of the individual sample (Chiu et al. 2018).
For biomarkers detected with at least 30% frequency, we estimated each participant’s exposure as the geometric mean of the biomarker concentrations measured in the composite samples collected during the intervention period. We then calculated the 25th, 50th, and 75th concentration percentiles in the organic and conventional groups, and compared these estimates using the nonparametric Mann-Whitney U test for non-normally distributed data.
3. Results
3.1. Study Population and Demographic Characteristics.
During the recruitment process, WIC clinic staff and counselors identified 49 potential study participants based on their initial screening for interest and eligibility. We attempted to contact all of these potential participants and ultimately enrolled 20 women from this group. Among those not enrolled, 13 were unable to be contacted and were unresponsive to messages, two were not interested in participating in the study, one had a miscarriage, and nine did not meet eligibility criteria (see Figure 1 for additional details). A total of 24 women provided informed consent, but four of these withdrew before the first food delivery or urine sample collection, either due to miscarriage (n=1) or because they were concerned about the time commitment of the study (n=3). Ultimately, 20 women enrolled and all of them completed the study. Of these, ten were randomly assigned to the conventional produce group and ten to the organic produce group.
Figure 1.
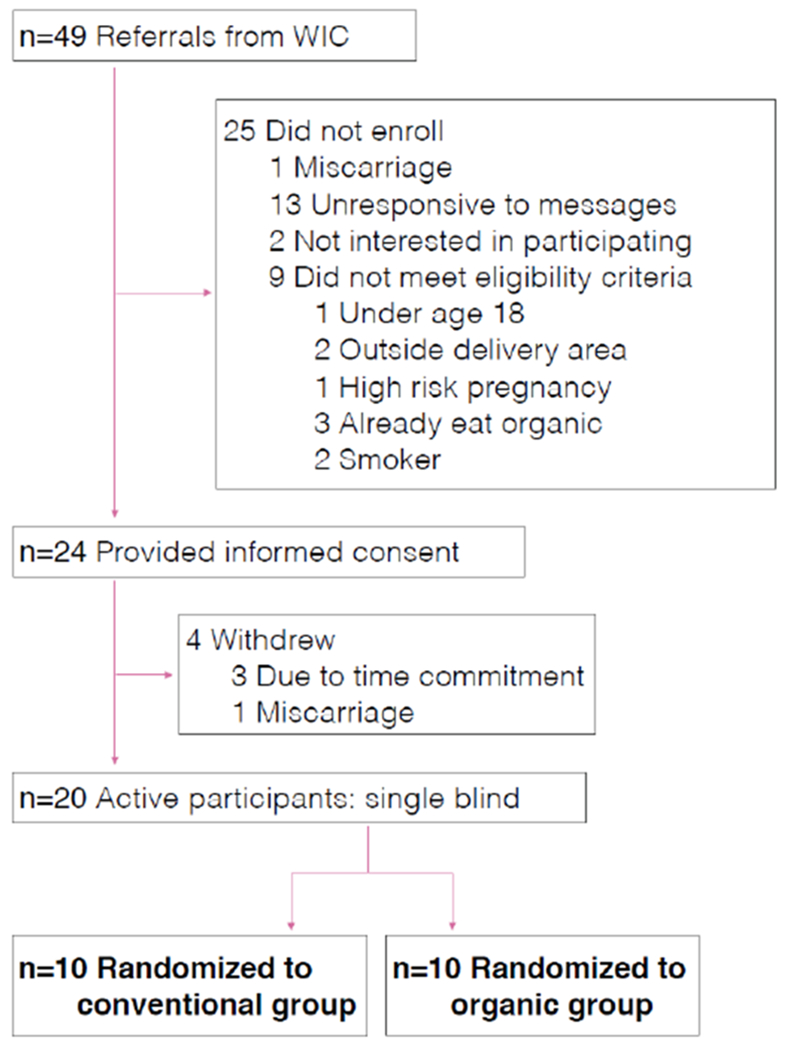
Details of participant recruitment and eligibility.
The average participant age was 27.8 years with a standard deviation of 5.4 years (Table 1). Participants in the organic produce group were slightly younger than those in the conventional produce group (26.4 ± 3.6 years vs 28.7 ± 6.7 years). Reflective of the overall demographics of the Treasure Valley of Idaho, the majority (75%) of study participants in the trial were Caucasian. In both groups, the median household income was between $20,000 and $29,999 per year. Participants randomized to the organic produce group were more highly educated than those randomized to the conventional produce group, with five having completed college or higher, compared to just two in the conventional group. This difference was not statistically significant, but is still important to note given the small sample size.
Table 1.
Participant demographics.
| All | Organic | Conventional | |
|---|---|---|---|
| n | 20 | 10 | 10 |
| Age at Enrollment (years, avg ± SD) | 27.8 ± 5.4 | 26.4 ± 3.6 | 28.7 ± 6.7 |
| Race/ethnicity (N) | |||
| Caucasian or White | 15 | 8 | 7 |
| African-American or Black | 1 | 0 | 1 |
| Hispanic/Latina | 4 | 2 | 2 |
| Highest level of education attained | |||
| Graduated high school | 4 | 1 | 3 |
| Some college | 9 | 4 | 5 |
| Bachelor’s degree | 6 | 4 | 2 |
| Graduate or other advanced degree | 1 | 1 | 0 |
| Household income ($ per year) | |||
| <$20,000 | 7 | 4 | 3 |
| $20,000 - $29,999 | 6 | 3 | 3 |
| $30,000 - $59,999 | 5 | 3 | 2 |
| $60,000 - $79,999 | 1 | 0 | 1 |
| Don’t know | 1 | 0 | 1 |
3.2. Pesticide Exposure.
Participants were asked a series of detailed questions about potential sources of residential pesticide exposure, including residential pesticide use by themselves, household members, or pest control companies. No participants in either study group reported any exposure to rodenticides, molluscicides, or fungicides. Three participants in the organic produce group and one participant in the conventional produce group reported occasional use of herbicides for weed control. One participant in the organic produce group and none in the conventional produce group reported use of flea or tick control for a pet. Five participants in the organic produce group and six participants in the conventional produce group reported that some insecticides had been used in their homes in the past year. Additional questions and product inspection indicated that none of these insecticides were organophosphates, but three of the participants in the organic produce group and one of the participants in the conventional produce group had used pyrethroid pesticides in their homes in the past year and were still storing those containers. Occupational pesticide exposure, either for the participant or any household members, was an exclusion criterion and as such, no participants reported that they or any members of their households were occupationally exposed to pesticides.
3.3. Food Diaries.
Seventeen of the twenty study participants elected to use the phone app to record their dietary choices; three used paper diaries. One of those using paper diaries was in the organic group and two were in the conventional group. Data from these food diaries indicated a significant difference between groups in the number of servings of organic produce consumed. Based on self-report, 66% of all produce consumed by participants in the “organic produce” group was organic (primarily indicated as “provided by the study”), compared to 2.7% in the conventional produce group (t-test, p<0.00001). Across both groups, the median number of servings of any produce items was two per day.
3.4. Urinary Biomonitoring.
Over the course of the trial, we collected a total of 461 individual spot urine samples, representing an average of 23 samples per participant. These included one individual pre-intervention spot sample per participant (total n=20), and 441 spot urine samples collected during the intervention period that were combined to yield a total of 116 monthly composites. Eighty-seven percent (87%) of the monthly composites were comprised of aliquots of all four corresponding weekly spot samples (average: 3.8 ± 0.6 spot samples per composite). Missed samples were primarily due to participants being out of town or delivering their babies earlier than 40-weeks gestation (sample collection ended at delivery).
Pesticide biomarker concentrations measured in the pre-intervention samples are difficult to compare to the samples analyzed during the intervention, as they represent single, individual spot samples as opposed to monthly composites. These samples therefore represent an exposure window of approximately 6-24 hours prior to their collection, and should not be interpreted as a representative measure of chronic pre-intervention exposure. Detection frequencies and concentrations measured in pre-intervention samples are shown in Table 2. Three of the metabolites were rarely detected in these pre-intervention samples. Specifically, 4-F-3-PBA was detected in the urine of one participant, and both IMPY and trans-DCCA were detected in the urine of two participants. Detection frequencies of the other metabolites were similar between participants who were randomized to receive conventional and organic produce, although participants who were randomized to the organic produce group were slightly more likely to have had detectable concentrations of urinary pesticide biomarkers prior to the intervention than those randomized to the conventional produce group. Specifically, nine of the participants randomized to the organic produce group had detectable urinary concentrations of 3-PBA prior to the intervention, compared to five in the conventional produce group. All ten participants randomized to the organic produce group had detectable urinary concentrations of PNP prior to the intervention, compared to eight of those randomized to receive conventional produce. TCPY was detected in pre-intervention samples from five participants in the organic produce group and four in the conventional produce group, and MDA was detected in pre-intervention samples from four participants in the organic produce group and two in the conventional produce group.
Table 2.
Frequency of detection of pyrethroid and organophosphate metabolites in pre-intervention samples.
| Metabolite | Parent Compounds | Detection Freauency | Median (μg/L) | 75th percentile (μg/L) | Max (μg/L) |
|---|---|---|---|---|---|
| Pyrethroids | |||||
| 3-PBA | Cyhalothrin, Cypermethrin, Deltamethrin, Fenproopathrin, Permethrin, Tralomethrin | 70% | 0.55 | 1.82 | 13.17 |
| trans-DCCA | Permethrin, Cypermethrin, Cyfluthrin | 10% | <LOD | <LOD | 20.43 |
| 4-F-3-PBA | Cyfluthrin, Flumethrin | 5% | <LOD | <LOD | 0.28 |
| Organophosphates | |||||
| PNP | Parathion, Methyl Parathion | 90% | 0.54 | 0.67 | 4.91 |
| TCPY | Chloroyrifos, Chloroyrifos-methyl | 45% | <LOD | 1.64 | 3.61 |
| MDA | Malathion | 35% | <LOD | 0.88 | 2.04 |
| IMPY | Diazinon | 10% | <LOD | <LOD | 0.75 |
Table 3 shows metabolite detection frequencies in composite samples collected during the intervention. Data are displayed both overall and separately for the organic and conventional groups. For all three pyrethroid biomarkers, detection frequencies were higher among participants receiving conventional produce compared to those receiving organic produce, although this difference was only statistically significant for trans-DCCA (3-PBA: 81% vs 68%, p=0.14; trans-DCCA: 16% vs 4%, p = 0.05; 4-F-3-PBA: 5% vs 4%, p = 1.0). For OP metabolites, all samples contained detectable concentrations of PNP. TCPY was detected more frequently in samples collected from women receiving conventional produce, although this difference was not statistically significant (39% vs 32%, p=0.44). Contrary to our hypothesis, both MDA and IMPY were detected more frequently in samples collected from women whose diets were supplemented with organic produce, although these differences were small and did not approach statistical significance (MDA: 20% vs 23%, p=0.82; IMPY: 7% vs 9%, p=0.76).
Table 3.
Frequency of detection of pyrethroid and organophosphate metabolites in composite urine samples collected during the dietary intervention.
| % Detected* | |||
|---|---|---|---|
| Metabolite | Overall (n=116) | Conventional (n=59) | Organic (n=57) |
| Pyrethroids | |||
| 3-PBA | 75% | 81% | 68% |
| trans-DCCA | 10% | 16%** | 4%** |
| 4-F-3-PBA | 4% | 5% | 4% |
| Organophosphates | |||
| PNP | 100% | 100% | 100% |
| TCPY | 35% | 39% | 32% |
| MDA | 22% | 20% | 23% |
| IMPY | 8% | 7% | 9% |
Our a priori decision to produce and compare summary statistics only for those metabolites with less than 70% censored data resulted in the exclusion of trans-DCCA, 4-F-3-PBA, MDA and IMPY from this component of the analysis. For the three remaining biomarkers, 3-PBA, PNP and TCPY, we calculated the geometric mean concentration for each of the 20 study participants from the concentrations measured in the six monthly composite samples, which represented approximately 22 spot urine samples per participant. Table 4 shows the median (50th percentile), as well as the 25th and 75th percentiles, of the geometric means of the concentrations measured in the monthly composite samples collected from each participant during the intervention period. Figure 2 shows these results graphically. While the range and 75th percentiles of the geometric mean concentrations were larger in the conventional group than the organic group for all three biomarkers, only 3-PBA was significantly different between the two groups (median of the geometric mean results: 0.95 μg/L vs 0.27 μg/L, p = 0.03).
Table 4.
For each participant, long-term exposure was estimated as the geometric mean of the concentrations in all monthly composite samples collected throughout the second and third trimesters of pregnancy. The distribution of these geometric mean concentrations in the organic and conventional groups is presented for all metabolites with less than 70% censored data.
| Percentiles (μg/L) | ||||
|---|---|---|---|---|
| 3-PBA* | n | 25th | 50th | 75th |
| Conventional | 10 | 0.54 | 0.95 | 1.85 |
| Organic | 10 | 0.23 | 0.27 | 0.80 |
| PNP | ||||
| Conventional | 10 | 0.39 | 0.50 | 0.76 |
| Organic | 10 | 0.47 | 0.50 | 0.57 |
| TCPY | ||||
| Conventional | 10 | 0.11 | 0.20 | 0.48 |
| Organic | 10 | 0.11 | 0.20 | 0.26 |
Concentrations in the conventional group were significantly higher than in the organic group (Mann-Whitney U test, p=0.03).
Figure 2.
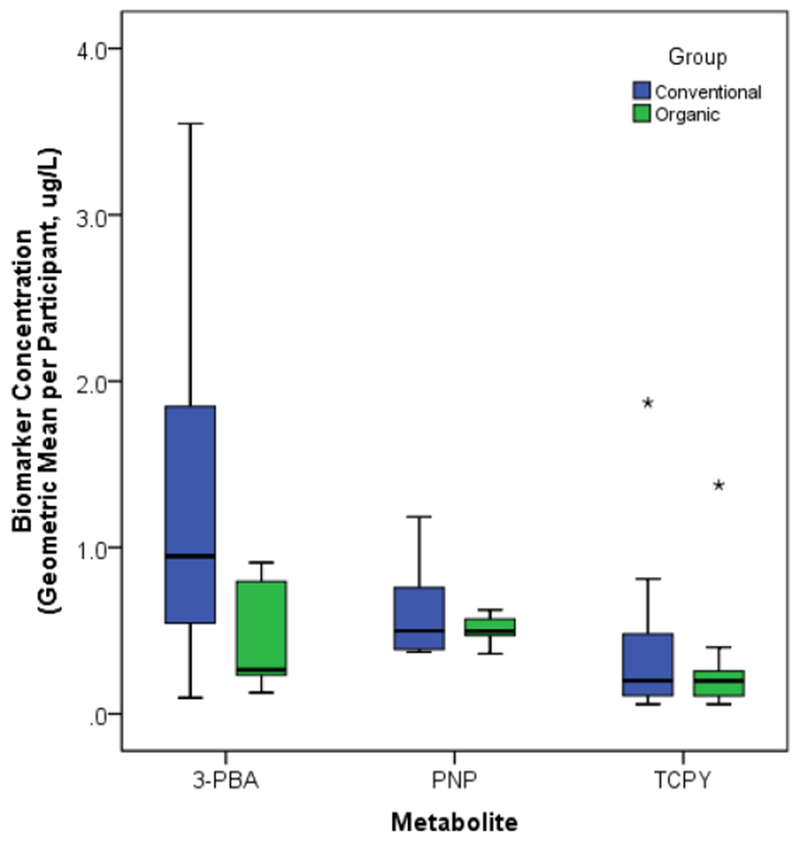
Distribution of participants’ long-term exposure, estimated as the geometric mean of the concentrations in all monthly composite samples collected throughout the second and third trimesters of pregnancy, by intervention group assignment. Boxes extend from the 25th to the 75th percentile, horizontal bars indicate the medians, whiskers extend 1.5 times the length of the interquartile range above and below the 75th and 25th percentiles, and outliers are represented as asterisks (*). 3-PBA concentrations were significantly higher among participants in the conventional as compared to the organic group (p=0.03); no significant difference was found for PNP or TCPY.
4. Discussion
We found that participants in the organic produce group had lower concentrations of urinary biomarkers of pyrethroid pesticide exposure than those in the conventional produce group. The most frequently detected pyrethroid biomarker in this study, 3-PBA, was found in significantly lower concentrations in urine samples collected from women receiving organic produce compared to those receiving conventional produce. The other two pyrethroid biomarkers examined in this study were not detected with sufficient frequency to allow a meaningful comparison of metabolite concentrations, but the detection frequency of trans-DCCA was significantly lower in samples collected from participants receiving organic produce compared to those receiving conventional produce. Together, these results suggest that addition of organic produce to an individual’s diet, as compared to conventional produce, significantly reduces exposure to pyrethroid insecticides. We did not observe this result for biomarkers of exposure to OP pesticides, whether evaluated based on detection frequency or urinary concentrations.
These results differ somewhat from results of previous studies, especially with respect to OP biomarkers. Several observational studies reported that children and adults with conventional diets had higher urinary concentrations of OP biomarkers than those with organic diets. In the first such study, conducted by members of our research group in 2001, urinary concentrations of the non-specific dialkylphosphate (DAP) biomarkers of OP exposure were found to be six times higher among 2-5 year old children whose parents reported that they consumed conventional diets compared to those whose parents reported feeding their children organic diets (Curl et al. 2003). More recently, researchers evaluating urinary concentrations of specific and non-specific OP metabolites found lower concentrations of dimethylphosphates among residents of a vegetarian community who reported higher intake of organic produce (Berman et al. 2016). Similarly, in a large multi-ethnic cohort of American adults recruited from six cities across the USA, we found DAP concentrations to be significantly lower among individuals who reported more frequent consumption of organic produce, after control for total fruit and vegetable intake (Curl et al. 2015).
Several experimental studies have supported the results of these observational investigations. Among 23 children aged 3-11 years, a five-day organic diet intervention significantly reduced urinary concentrations of specific biomarkers of OP pesticide exposure (Lu et al. 2006b; Lu et al. 2008). In this study, the researchers substituted many categories of conventional items with organic versions, including fresh and processed fruits and vegetables, juices, and some wheat- and corn-based items. In another interventional study in 2006, Bradman et al. (2015) recruited a group of 40 Mexican-American children aged 3-6 years, 20 of whom lived in an urban community and 20 of whom lived in a predominantly agricultural community. Regardless of residential location, introduction of a 7-day organic diet significantly reduced DAP concentrations in the children’s urine. In this study, the researchers substituted several food categories, including fruits, breads, cereals, vegetables, dairy, eggs, juices and snacks. A crossover study of 13 non-occupationally exposed adults in Australia also found urinary DAP concentrations to be significantly lower when the participants were asked to consume as close to a 100% organic food diet as possible for seven days (Oates et al. 2014). Most recently, Hyland et al. (2019) reported significant reductions in urinary levels of several pesticide metabolites – with some of the most marked decreases among specific metabolites of OPs – among four families whose conventional diets were replaced with fully organic diets.
In our study, we did not find a significant difference between concentrations of OP biomarkers in the conventional and organic produce groups. Unlike many of these previous studies, we did not attempt to substitute a fully organic diet for the participants’ conventional diets, instead focusing on fresh fruit and vegetable items. OP and pyrethroid insecticides are more commonly used on fruit and vegetable crops than livestock, dairy or grain, but it is possible that our results may have underestimated the effect of an organic diet on OP exposure because we did not include processed fruits and vegetables or juices in the dietary intervention. We also did not measure the non-specific DAP metabolites, which represent collective exposure to many OP pesticides, and so our results are limited to the specific biomarkers of a much smaller set of parent compounds. Of the OP biomarkers investigated in this current study, one – PNP – represents exposure to parathion and methyl parathion (neither of which is currently registered for use in American agriculture), but is also itself used to manufacture drugs and dyes (Qiao et al. 2000). The finding that 100% of our samples contained detectable concentrations of PNP may not be informative as to differences in pesticides exposure. For TCPY, the specific biomarker of exposure to chlorpyrifos, we did see a larger range and a higher 75th percentile for concentrations of this biomarker in the conventional produce group compared to the organic produce group. However, the median concentrations were nearly identical, and this difference was not statistically significant. MDA and IMPY, the specific biomarkers of malathion and diazinon, were infrequent and not different between the two groups.
It is also possible that, with regard to OP pesticides, the difference between our findings and those of previous studies may be related to changes in agricultural pesticide use practices over time. The dietary interventions conducted by Lu et al. (2006b; 2008) and Bradman et al. (2015), described above, occurred during the early to mid-2000s. In the ten to fifteen years since those studies were conducted, OP pesticide use in agriculture has decreased, and pyrethroid use has risen (Palmquist et al. 2012). At the same time, concentrations of many OP pesticide metabolites in the overall population have dropped while many pyrethroid metabolite concentrations have increased (CDC, 2018). Thus, it could be that an organic diet may not have as significant an effect on OP exposure as it may have had in the previous decade, although the recent findings by Hyland et al. (2019) do not support this hypothesis.
With respect to pyrethroids, participants in the organic produce group had significantly lower urinary biomarkers of exposure than participants in the conventional produce group. The results of previous studies of the effect of an organic diet on pyrethroid exposure have been somewhat mixed, compared to previous studies of OP exposure. Bradman et al. (2015) found urinary concentrations of 3-PBA to decrease following an organic diet intervention among children living in Oakland, CA, but not among those living in the Salinas Valley, CA. And while Lu and colleagues (2006a) reported a dramatic reduction in OP exposure following an organic diet intervention in 23 elementary school children, they found residential pesticide use to be more important than diet as a predictor of pyrethroid metabolite concentrations in children’s urine. More recently, Hyland et al. (2019) reported decreases of 43 to 57% for the metabolites of multiple pyrethroid insecticides (including 3-PBA and trans-DCCA) following a fully organic diet intervention. Changes in pesticide use patterns over time, and particularly the increased use of pyrethroids in agriculture described above, may explain the less ambiguous effect of an organic intervention on pyrethroid exposure observed in both the recent work described by Hyland el al. (2019) and the current study compared to earlier research.
To our knowledge, this is the first study to evaluate the effect of a long-term organic diet intervention. All previous studies have included interventions lasting one to two weeks; this study evaluated the effect of a 24-week intervention. Our biomonitoring approach, which included collection of an average of 23 urine samples per participant, was also sufficiently intensive to capture and characterize long-term exposure. Thus, for the first time, we are able to evaluate the effect of an organic diet intervention on pesticide exposures on a more chronic timescale. This also is the first study to investigate the effect of an organic diet intervention on pesticide exposure among pregnant women. Neuroscientific research has identified embryonic and fetal development to be among the “critical windows of vulnerability” to neurotoxic chemicals (Bennett et al. 2016), and thus pregnant women are an important population to consider when evaluating potential opportunities for exposure reduction.
Finally, instead of providing study participants with a fully organic diet and encouraging strict compliance, we designed an intervention more closely related to actual dietary consumption patterns. Previous researchers’ decisions to evaluate the effect of a fully organic diet was clearly appropriate for the goal of understanding the contribution of the dietary pathway to pesticide exposure. One of our goals, however, was to determine whether it would be feasible to conduct a longer-term organic diet intervention study that would be acceptable to participants. In this way, this work provides preliminary data for a future investigation of whether organic food consumption is associated with improved neurocognitive outcomes in children. For that future work to be meaningful, the dietary intervention must be realistic. Consumption of a fully organic diet is rare; in our previous research, among a sample of more than 4,000 adults recruited from across the USA, we found that fewer than 5% reported that they “often or always” consumed organic food, whereas 35% reported that they “sometimes” did (Curl et al. 2013). Thus, the dietary intervention we selected for this study was intended to better reflect real-world dietary habits.
While there are several important and novel attributes to this study, there are also areas of remaining uncertainty. Use of urinary biomarkers to assess pesticide exposure is limited by the possibility that measured concentrations may represent exposure to preformed metabolites rather than the parent compounds themselves (Sudakin and Stone 2011), and no data were available regarding residue levels of parent compounds on produce samples. This could result in overestimation of the potential effect of the dietary intervention on any health outcomes. This fact is less of a concern in the current study because we are not evaluating health effects, but it could be problematic for future epidemiological inference. In addition, several of the participants in this study did report residential use of pyrethroid pesticides and containers of these pesticides were found in their homes. Because the frequency of residential pyrethroid use was similar in the organic and conventional groups, we do not think that this affected the study results. However, we cannot rule out the possibility that residential sources, in addition to dietary sources, contributed to the measured biomarker concentrations.
This study had a small sample size of twenty participants, although 461 spot urine samples were collected and analyzed as 116 monthly composites. A larger number of participants would have generated more precise exposure estimates, which may have allowed us to see differences in biomarkers of OP pesticides between the organic and conventional produce groups – if they existed – that were not apparent in these data. Finally, participants in this study were relatively homogenous in terms of race/ethnicity and income levels. While these variables should not necessarily affect our conclusions in this study, a more diverse population may be more appropriate for future research regarding the relationship between organic food consumption and health effects. In terms of education, participants in the organic produce group had somewhat higher educational attainment than those in the conventional produce group. Because higher educational attainment is frequently associated with greater intake of fruits and vegetables, and fruit and vegetable intake has been associated with pesticide exposure, this difference could dampen any relationship between assignment to the organic produce group and reduced pesticide exposure. This could potentially bias results towards the null and would result in an underestimation of the effect of organic produce consumption on pesticide exposure in this cohort. We believe this is unlikely, however, since the median intake of fruits and vegetables was two servings per day in both groups.
Despite these limitations, this study provides evidence that addition of organic, rather than conventional, produce to an individual’s diet may be an effective strategy to reduce exposure to pyrethroid pesticides. This work demonstrates that a fully, 100% organic diet is not required to see a significant reduction in urinary biomarkers of exposure to these insecticides. Finally, this study, which included the collection of many more biological samples than in previous studies, demonstrates the feasibility of a long-term organic diet intervention in a potentially vulnerable population and provides an estimate of the effect of such an intervention on exposure to pyrethroid and OP pesticides.
Highlights.
A long-term (24-week) organic produce intervention among pregnant women is feasible
Participants who were provided weekly deliveries of organic produce had significantly lower urinary levels of biomarkers of exposure to pyrethroid insecticides compared to those who were provided conventional produce
No statistically significant differences in detection frequency or concentrations were found for any of the four biomarkers of OP exposure quantified in this trial
Acknowledgments:
Research reported in this publication was supported by Grant UL1 TR002319 from the NIH National Center for Advancing Translational Sciences through the Clinical and Translational Science Awards Program (CTSA) and by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number K01ES028745. The content of this manuscript, including all findings and conclusions, is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the official position of the Centers for Disease Control and Prevention. The research team would like to thank the women who generously shared their time to participate in this research. The authors also gratefully acknowledge Mark Davis, Dickson Wambua, William Roman, Isuru Vidanage, and Meghan Vidal (CDC, Atlanta, GA) for their technical assistance with pesticide biomarkers measurements, and Makaela Bournazian, Dana Kerins, Hope Murray, Justin Nelson, Abigail Thomas and Jonathan Wheatley (Boise State University, Boise, ID) for their assistance in sample collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or competing financial interests.
References
- Antweiler RC, Taylor HE. 2008. Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary statistics. Environ Sci Technol 42(10),3732–8. [DOI] [PubMed] [Google Scholar]
- Baker BP, Benbrook CM, Groth E 3rd, Lutz Benbrook K. 2002. Pesticide residues in conventional, integrated pest management (IPM)-grown and organic foods: insights from three US data sets. Food Additives & Contaminants 19(5),427–46. [DOI] [PubMed] [Google Scholar]
- Bennett D, Bellinger DC, Birnbaum LS, Bradman A, Chen A, Cory-Slechta DA, et al. Association. 2016. Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement. Environmental Health Perspectives 124(7),A118–A22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman T, Göen T, Novack L, Beacher L, Grinshpan L, Segev D, et al. 2016. Urinary concentrations of organophosphate and carbamate pesticides in residents of a vegetarian community. Environ Int 96,34–40. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119(8),1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Quirós-Alcalá L, Castorina R, Aguilar Schall R, Camacho J, Holland NT, et al. 2015. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect 123(10),1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier C, Warembourg C, Le Maner-Idrissi G, Lacroix A, Rouget F, Monfort C, et al. 2016. Organophosphate Insecticide Metabolites in Prenatal and Childhood Urine Samples and Intelligence Scores at 6 Years of Age: Results from the Mother–Child PELAGIE Cohort (France). Environmental Health Perspectives 124(5),674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL. 2008. Multi-rule quality control for the age-related eye disease study. Stat Med 27:4094–4106. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2018. Fourth national report on human exposure to environmental chemicals. Updated Tables, March 2018, Volume 1 Available at: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf/. Accessed: 11/12/18. [Google Scholar]
- Chiu YH, Williams PL, Minguez-Alarcón L, Gillman M, Sun Q, Ospina M, et al. 2018. Comparison of questionnaire-based estimation of pesticide residue intake from fruits and vegetables with urinary concentrations of pesticide biomarkers. J Expo Sci Environ Epidemiol 28(1),31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Fenske RA, Elgethun K. 2003. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ Health Perspect, 111(3),377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Beresford SA, Hajat A, Kaufman JD, Moore K, Nettleton JA, et al. 2013. Associations of organic produce consumption with socioeconomic status and the local food environment: Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 8(7),e69778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Beresford SA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, et al. 2015. Estimating pesticide exposure from dietary intake and organic food choices: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 123(5),475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Wade EL, Restrepo PR, Roman-Esteva W, Bravo R, Kuklenyik P, et al. 2013. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 929,18–26. [DOI] [PubMed] [Google Scholar]
- Dettmann RL, Dimitri C. 2009. Who’s buying organic vegetables? Demographic characteristics of US consumers. Journal of Food Products Marketing 16(1),79–91. [Google Scholar]
- Donauer S, Altaye M, Hu Y, Sucharew H, Succop P, Calafat AM, et al. 2016. An observational study to evaluate associations between low-level gestational exposure to organophosphate pesticides and cognition during early childhood. Am J Epidemiol 284(5), 410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. 2011. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 119(8),1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Bradman A, Wolff MS, Rauh VA, Harley KG, Yang JH, et al. 2016. Prenatal Organophosphorus Pesticide Exposure and Child Neurodevelopment at 24 Months: An Analysis of Four Birth Cohorts. Environ Health Perspect 124(6),822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency). 2017. Pesticide Industry Sales and Usage, 2008-2012 Market Estimates. Available: https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf. Accessed 5/15/19.
- Eskenazi B, Bradman A, Castorina R. 1999. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect 107(Suppl 3),409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Kogut K, Huen K, Harley KG, Bouchard M, Bradman A, et al. 2014. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res 134,149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong MA, Barr DB, Wolff MS, Engel SM. 2017. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 62,231–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göen T, Schmidt L, Lichtensteiger W, Schlumpf M. 2017. Efficiency control of dietary pesticide intake reduction by human biomonitoring. Int J Hyg Environ Health 220(2 Pt A),254–60. [DOI] [PubMed] [Google Scholar]
- González-Alzaga B, Lacasaña M, Aguliar-Garduño C, Rodriguez-Barranco M, Ballester F, Rebagliato M, et al. 2014. A systematic review of neurodevelopmental effects of prenantal and postnatal organophosphate pesticide exposure. Toxicol Lett 230(2):104–21. [DOI] [PubMed] [Google Scholar]
- Govindasamy R, Italia J. 1999. Predicting willingness-to-pay a premium for organically grown fresh produce. Journal of Food Distribution Research 30,44–53. [Google Scholar]
- Hernández AF, González Alzaga, López-Flores I, Lacasaña M. 2016. Systematic reviews on neurodevelopmental and neurodegenerative disorders linked to pesticide exposure: Methodological features and impact on risk assessment. Environ Int 92-93: 657–79. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Sass JB, Engle S, Bennett DH, Bradman A, Eskenzai B, et al. 2018. Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms. PLoS Med 15(10): el002671 10.1371/journal.pmed.1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 5(1),46–51. [Google Scholar]
- Hu Y, Chiu YH, Hauser R, Chavarro J, Sun Q. 2016. Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: A validation study. Environ Int 92-93,294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland C, Bradman A, Gerona R, Patton S, Zakharevich I, Gunier RB, et al. 2019. Organic diet intervention significantly reduces urinary pesticide levels in U.S. children and adults. Environ Res 171:568–575. [DOI] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA. 2008. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect 116(4),537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Bartell S, Bravo R. 2006a. A longitudinal approach to assessing urban and suburban children’s exposure to pyrethroid pesticides. Environ Health Perspect 114(9),1419–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Walker LA, Bravo R. 2009. The attribution of urban and suburban children’s exposure to synthetic pyrethroid insecticides: a longitudinal assessment. J Expo Sci Environ Epidemiol 19(1),69–78. [DOI] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. 2006b. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect 114(2),260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect 118(12),1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk LJ, Xue J, Brown GG, McCombs M, Nishioka M, Michael LC. 2014. Dietary intakes of pesticides based on community duplicate diet samples. Sci Total Environ 468-469,785–90. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, et al. 2013. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotox 39:158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougadère A, Sirot V, Kadar A, Fastier A, Truchot E, Vergnet C, et al. 2012. Total diet study on pesticide residues in France: levels in food as consumed and chronic dietary risk to consumers. Environ Int 45,135–50. [DOI] [PubMed] [Google Scholar]
- Oates L, Cohen M. 2011. Assessing diet as a modifiable risk factor for pesticide exposure. Int J Environ Res Public Health 8(6),1792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates L, Cohen M, Braun L, Schembri A, Taskova R. 2014. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res 132,105–11. [DOI] [PubMed] [Google Scholar]
- Palmquist K, Salatas J, Fairbrother A. 2012. Pyrethroid Insecticides: Use, Environmental Fate, and Ecotoxicology. in Perveen F (ed.), Insecticides - Advances in Integrated Pest Management. [Google Scholar]
- Petersen SB, Rasmussen MA, Strøm M, Halldorsson TL, Olsen SF. 2013. Sociodemographic characteristics and food habits of organic consumers – a study from the Danish National Birth Cohort. Public Health Nutr. 16(10):1810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C, Chevrier C, Durand G, Monfort C, Rouget F, Garlantezec R, et al. 2010. Impact on fetal growth of prenatal exposure to pesticides due to agricultural activities: a prospective cohort study in Brittany, France. Environmental Health 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao GL, Chang SK, Brooks JD, Riviere JE. 2000. Dermatotoxicokinetic Modeling of p-Nitrophenol and Its Conjugation Metabolite in Swine following Topical and Intravenous Administration. Toxicological Sciences 54(2),284–94. [DOI] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano AM, et al. 2012. Associations of Prenatal Exposure to Organophosphate Pesticide Metabolites with Gestational Age and Birth Weight. Environmental Health Perspectives 120(7),1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, Reigart JR (eds.). 2013. Recognition and Management of Pesticide Poisoning (6th edn., Washington, D.C.: Environmental Protection Agency (EPA)). [Google Scholar]
- Sagiv SK, Harris MH, Gunier RB, Kogut KR, Harley KG, Deardorff J, et al. 2018. Prenatal Organophosphate Pesticide Exposure and Traits Related to Autism Spectrum Disorders in a Population Living in Proximity to Agriculture. Environ Health Perspect 126(4),047012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Wüst AP, Moltó-Puigmartí C, Jansen EH, van Dongen MC, Dagnelie PC, Thijs C. 2017. Organic food consumption during pregnancy and its association with health-related characteristics: the KOALA Birth Cohort Study. Public Health Nutr. 20(12): 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TA, Huang CL, Lin B. 2009. Does price or income affect organic choice? Analysis of US fresh produce users. Journal of Agricultural and Applied Economics 41(03),731–44. [Google Scholar]
- Stein LJ, Gunier RB, Harley K, Kogut K, Bradman A, Eskenazi B. 2016. Early childhood adversity potentiates the adverse association between prenatal organophosphate pesticide exposure and child IQ: The CHAMACOS cohort. Neurotoxicology 56,180–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin DL, Stone DL. 2011. Dialkyl phosphates as biomarkers of organophosphates: The current divide between epidemiology and clinical toxicology. Clinical Toxicology 49(9),771–81. [DOI] [PubMed] [Google Scholar]
- USDA (U.S. Department of Agriculture). 2011. Pesticide Data Program. Washington, DC: Available at: http://www.ams.usda.gov/datasets/pdp. Accessed: 11/12/18. [Google Scholar]
- USDA (U.S. Department of Agriculture). 2017. Pesticide Data Program. Washington, DC: Available at: http://www.ams.usda.gov/datasets/pdp. Accessed: 5/16/19. [Google Scholar]
- USDA (U.S. Department of Agriculture). 2017. Economic Research Service – Organic Market Overview. Available at: https://www.ers.usda.gov/topics/natural-resources-environment/organic-agriculture/organic-market-overview.aspx. Accessed: 5/16/19.
- US EPA (U.S. Environmental Protection Agency). 2000. ‘Chlorpyrifos. Revised risk assessment and agreement with registrants.Fed Reg 66:7753–7759. [Google Scholar]
- US EPA. 2001. Diazinon revised risk assessment and agreement with registrants. Fed Reg 69:48864–48867. [Google Scholar]
- Viel JF, Rouget F, Warembourg C, Monfort C, Limon G, Cordier S, et al. 2017. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: the PELAGIE mother-child cohort. Occup Environ Med 74(4),275–81. [DOI] [PubMed] [Google Scholar]
- Viel JF, Warembourg C, Le Maner-Idrissi G, Lacroix A, Limon G, Rouget F, et al. 2015. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: The PELAGIE mother-child cohort. Environ Int 82,69–75. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. 2004. Prenatal Insecticide Exposures and Birth Weight and Length among an Urban Minority Cohort. Environmental Health Perspectives 112(10),1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PR, Hammitt JK. 2001. Perceived risks of conventional and organic produce: pesticides, pathogens, and natural toxins. Risk analysis 21(2),319–30. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel S, Berkowitz G, Teitelbaum S, Siskind J, Barr DB, et al. 2007. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res 61(2),243–50. [DOI] [PubMed] [Google Scholar]
- Zhang F, Huang CL, Lin B, Epperson JE. 2008. Modeling fresh organic produce consumption with scanner data: a generalized double hurdle model approach. Agribusiness: An International Journal 24(4),510–22. [Google Scholar]


