Figure 2: Assessment of PN Using PRO and CTCAE.
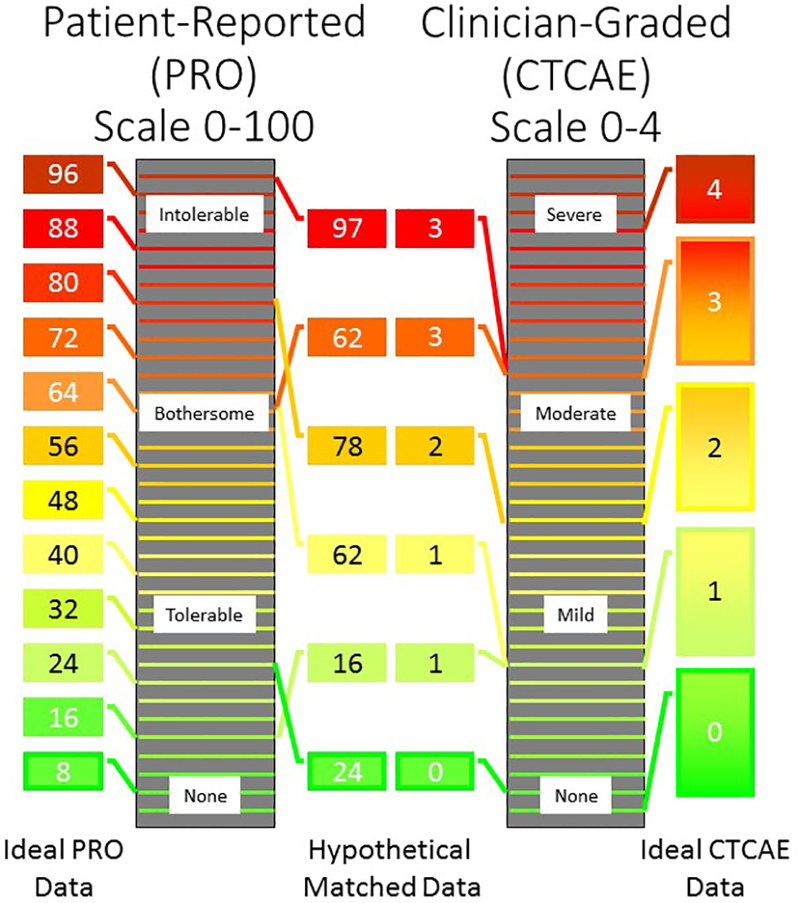
Schematic representation of the PRO (left) and CTCAE (right) grading scales for peripheral neuropathy (PN). The height of the box represents the extent of PN and the number inside the box is the tolerability assigned by the patient (left) or severity assigned by the clinician (right). Ideally, all patients (far left) and clinicians (far right) would have consistent scales, in which case PRO would likely be a superior endpoint for biomarker research due to its increased sensitivity. However, there is evidence that thresholds of severity are more consistent for clinicians (inner right) than patients (inner left)15. For example, two patients rated their PN a 62 on a scale of 0–100, but the actual extent of PN was very different between these patients. This increased inter-rater variability could be a critical drawback for using PRO as an endpoint in PN biomarker studies.
