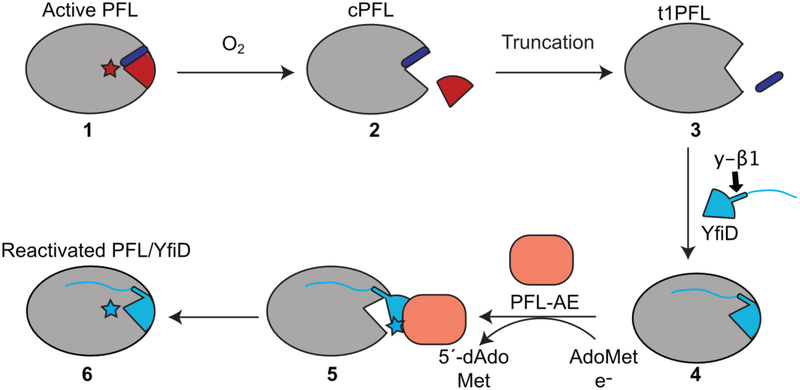
Figure 8:
A revised model for YfiD rescue. When exposed to oxygen, activated PFL is cleaved at its glycyl radical site, G734 (cPFL). The cleaved region of PFL (G734 through the C-terminus) is depicted in red. In order for YfiD to fit into the pocket, additional residues that include residues of βlO strand of PFL (purple) must be lost, forming a tIPFL variant. From here, we propose that the y-βl strand of YfiD completes the 10-stranded barrel of PFL and the disordered N-terminus of YfiD helps secure this spare part protein to tIPFL. After YfiD radical domain (GRD) flips out, allowing PFL-AE to activate YfiD. Once activated, the GRD of YfiD enters the open PFL active site, resulting in a reactivated PFL:YfiD complex. This complex should remain stable for multiple rounds of turnover with YfiD acting as a noncovalent subunit.