Abstract
Classical Hodgkin lymphoma (cHL) patients with relapsed or refractory disease may benefit from allogeneic hematopoietic cell transplantation (allo-HCT), but many lack a matched sibling donor (MSD). Herein, we compare outcomes of two reduced-intensity conditioning (RIC) HCT platforms in cHL: T cell-replete related donor haploidentical (haplo) HCT with post-transplantation cyclophosphamide (PTCy)-based approach versus MSD/calcineurin inhibitor (CNI)-based approach. The study included 596 adult patients who underwent a first RIC allo-HCT for cHL between 2008-2016, using either haplo-PTCy (n=139) or MSD/CNI-based (n=457) approach. Overall survival (OS) was the primary endpoint. Secondary endpoints included acute (a) and (c) graft-versus-host disease (GVHD), non-relapse mortality (NRM), relapse/progression, and progression-free survival (PFS). On multivariate analysis, there was no significant difference between haplo/PTCy and MDS/CNI-based approaches in terms of OS (hazard ratio [HR]=1.07; 95%CI=0.79-1.45; p=0.66) or PFS (HR=0.86; 95%CI=0.68-1.10; p=0.22). Haplo/PTCy was associated with a significantly higher risk of grade 2-4 aGVHD (odds ratio [OR]=1.73, 95%CI=1.16-2.59, p=0.007), but the risk of grade 3-4 aGVHD was not significantly different between the two cohorts (OR=0.61, 95%CI=0.29-1.27, p=0.19). The haplo/PTCy platform provided a significant reduction in cGVHD risk (HR=0.45, 95%CI=0.32-0.64, p<0.001), and a significant reduction in relapse risk (HR=0.74, 95%CI=0.56-0.97, p=0.03). There was a statistically non-significant trend towards higher NRM with haplo/PTCy approach (HR=1.65, 95%CI=0.99-2.77, p=0.06). Haplo/PTCy-based approaches are associated with lower incidence of cGVHD and relapse, with PFS and OS outcomes comparable to MSD/CNI-based approaches. There was a leaning towards higher NRM with haplo/PTCy-based platform. These data show that haplo/PTCy allo-HCT in cHL results in survival comparable to MSD/CNI-based allo-HCT.
Keywords: Hodgkin lymphoma, haploidentical transplantation, allogeneic transplantation, alternative donor
INTRODUCTION
Classical Hodgkin lymphoma (cHL) patients with relapsed/refractory disease may benefit from allogeneic hematopoietic cell transplantation (allo-HCT). Classica HL patients who relapse after an autologous HCT have poor outcomes, with a 5-year overall survival (OS) of ~30%.[1, 2] Although in theory, myeloablative conditioning (MAC) could improve disease control going into allo-HCT, these higher intensity approaches in allo-HCT for cHL have generally been associated with higher rates of non-relapse mortality (NRM).[3-5] Reduced-intensity conditioning (RIC) regimens have extended the use of allo-HCT to those who relapse after autologous HCT, older patients, and those with significant comorbidities.[6-8] In a disease for which immunotherapy has shown great promise, the application of cellular immunotherapy in the form of allo-HCT will likely remain a critical component of cHL therapeutics for the foreseeable future. Currently there remains an ongoing risk of relapse in patients treated with programmed cell death protein 1 (PD-1) blockade as monotherapy and there are no conclusive data that such immunotherapy is curative for majority of relapsed/refractory cHL. In addition, there are concerns for increased toxicity with allo-HCT in those treated with PD-1 inhibitors, with the majority of patients who will go on to allo-HCT in the future, will likely have exposure to such agents. Thus, comparing outcomes across different RIC HCT approaches will serve to better inform how to maximize the curative potential of allo-HCT while also assessing the impact of NRM and graft-versus-host disease (GVHD).
In a significant proportion of patients requiring an allo-HCT, a conventional matched donor is not available and several reports now show that T-cell replete related donor haploidentical (haplo) HCT with post-transplantation cyclophosphamide (PTCy) is a suitable option for patients with relapsed/refractory cHL with similar survival outcomes and lower rates of chronic GVHD, compared to matched sibling donors (MSD) and matched unrelated donors (MUD).[5, 9-12] Intriguingly, some small studies have suggested that haplo HCT may be associated with lower risk of relapse and improved progression-free survival (PFS) when compared to MSD HCT. [5, 13]
In this study we use a large registry dataset to examine the outcomes of two RIC platforms for HCT: the haplo-PTCy-based approach compared to MSD/calcineurin inhibitor (CNI)-based approach in patients with cHL.
PATIENTS AND METHODS
Data source
Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of more than 500 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin. Participating centers are required to report all transplantations consecutively; patients are followed longitudinally, and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. The CIBMTR collects data at two levels, transplant essential data (TED) in all patients and more comprehensive data (CRF) in a subset of patients selected by a weighted randomization scheme. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Patients
Included in this analysis are adult (≥18 years) cHL patients undergoing their first non-myeloablative or RIC (NMA/RIC) allo-HCT, between 2008 and 2016. This was a comparison of two HCT approaches, with eligible patients either receiving a T-cell replete related donor PTCy-based haplo graft (haplo/PTCy-based) (± CNI and mycophenolate mofetil [MMF]) or MSD grafts with CNI-based GVHD prophylaxis (MSD/CNI-based). MSD recipients could have received antithymocyte globulin (ATG) or alemtuzumab. Patients receiving ex vivo graft manipulation were not included.
Definitions & Study Endpoints
The intensity of allo-HCT conditioning regimens was categorized as NMA/RIC using consensus criteria.[14] Disease response at time of HCT was determined using the International Working Group criteria in use during the era of this analysis.[15] The primary endpoint was OS; death from any cause was considered an event and surviving patients were censored at last follow up. Secondary outcomes included NRM, progression/relapse, and PFS. NRM was defined as death without evidence of lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. Acute GVHD and chronic GVHD were graded using established clinical criteria.[16, 17] Probabilities of PFS and OS were calculated using the Kaplan–Meier estimates. Neutrophil recovery was defined as the first of 3 successive days with ANC >500/μL after post-transplantation nadir. Platelet recovery was considered to have occurred on the first of three consecutive days with platelet count 20,000/μL or higher, in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical analysis
The haplo/PTCy cohort was compared against MSD/CNI cohort. Cumulative incidences of hematopoietic recovery, GVHD, relapse, and NRM were calculated to accommodate for competing risks. Associations among patient-, disease, and transplantation-related variables and outcomes of interest were evaluated using Cox proportional hazards regression for chronic GVHD, relapse, NRM, PFS, and OS and logistic regression for acute GVHD. Forward stepwise selection was used to identify covariates that influenced outcomes. Covariates with a p<0.05 were considered significant. The proportional hazards assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Interactions between the main effect and significant covariates were examined. Center effect was tested using the score test for chronic GVHD, relapse, NRM, PFS, and OS and the generalized linear mixed model for acute GVHD.[18] Results are expressed as odds ratio (OR) for acute GVHD and hazard ratio (HR) for chronic GVHD, relapse, NRM, PFS, and OS. The variables considered in multivariate analysis are shown in Table 1S of supplemental appendix. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS:
Baseline Characteristics:
Five hundred and ninety-six adult cHL patients undergoing their first NMA/RIC allo-HCT between 2008-2016 and reported to CIBMTR were included. Among these 139 received a RIC haplo/PTCy-based approach while 457 received a RIC MSD/CNI-based approach. A higher proportion of recipients in the haplo/PTCy group received bone marrow (BM) as a graft source (70% vs 4%) and were of African American ethnicity (19% vs 5%). A higher proportion of MSD/CNI cohort received a prior autologous HCT compared to haplo/PTCy cohort (84% vs. 73%). Fourteen percent of MSD/CNI group received ATG or alemtuzumab compared to less than 1% in the haplo/PTCy group. Baseline patient-, disease-, and transplantation-related characteristics are shown in Table 1.
Table 1.
Baseline characteristics of NMA/RIC conditioning matched sibling donor or haploidentical donor patients with HL registered to the CIBMTR from 2008-2016.
| Matched Sibling Donor |
Haploidentical Donor |
P-value | |
|---|---|---|---|
| Number of patients | 457 | 139 | |
| Number of centers | 131 | 44 | |
| Median patient age, years (range) | 33 (18-66) | 33 (19-69) | 0.92 |
| Male gender | 257 (56) | 81 (58) | 0.67 |
| Patient race | <0.001 | ||
| Caucasian | 371 (81) | 102 (73) | |
| African American | 23 (5) | 27 (19) | |
| Other1 | 61 (13) | 9 (6) | |
| Missing | 2 (<1) | 1 (<1) | |
| Karnofsky performance score ≥ 90 | 343 (75) | 103 (74) | 0.63 |
| Missing | 15 (3) | 7 (5) | |
| HCT-CI | 0.01 | ||
| 0 | 194 (42) | 44 (32) | |
| 1-2 | 101 (22) | 45 (32) | |
| ≥3 | 133 (29) | 46 (33) | |
| Missing | 29 (6) | 4 (3) | |
| Previous autologous HCT | 382 (84) | 102 (73) | 0.007 |
| Median time from diagnosis to transplant, months | 34 (4-338) | 32 (8-236) | 0.02 |
| Remission at HCT | 0.72 | ||
| Complete remission | 178 (39) | 45 (32) | |
| Partial remission | 195 (43) | 65 (47) | |
| Resistant disease | 74 (16) | 26 (19) | |
| Untreated relapse | 6 (1) | 2 (1) | |
| Unknown | 4 (<1) | 1 (<1) | |
| Conditioning regimens2 | <0.001 | ||
| Flu/Mel | 187 (41) | 7 (5) | |
| Flu/Cy/TBI | 21 (5) | 122 (88) | |
| Others | 249 (54) | 10 (7) | |
| Conditioning intensity | <0.001 | ||
| NMA | 137 (30) | 125 (90) | |
| RIC | 320 (70) | 14 (10) | |
| TBI dose | <0.001 | ||
| 200 cGy | 60 (13) | 123 (88) | |
| > 200 cGy | 18 (4) | 1 (<1) | |
| No TBI given | 379 (82) | 15 (11) | |
| Graft type | <0.001 | ||
| Bone marrow | 18 (4) | 97 (70) | |
| Peripheral blood | 439 (96) | 42 (30) | |
| Number of patients | 457 | 139 | |
| GVHD prophylaxis | <0.001 | ||
| Post-CY ± other(s) | 0 | 1393 | |
| CNI + MMF based | 142 (31) | 0 | |
| CNI + MTX based | 219 (48) | 0 | |
| CNI ± other(s) (except MMF, MTX, PTCy) | 96 (21) | 0 | |
| ATG or alemtuzumab use | 66 (14) | 1 (<1) | <0.001 |
| Donor/recipient gender | 0.090 | ||
| Female → Male | 111 (24) | 38 (27) | |
| Others | 346 (76) | 101 (73) | |
| CMV status D+/R− | 0.025 | ||
| +/− | 65 (14) | 29 (21) | |
| Other | 397 (83) | 109 (79) | |
| Missing | 13 (3) | 1 (<1) | |
| Year of transplant | 0.001 | ||
| 2008-2010 | 162 (35) | 38 (27) | |
| 2011-2013 | 187 (41) | 40 (29) | |
| 2014-2016 | 108 (24) | 61 (44) | |
| Median follow-up of survivors (range), months | 52 (2-101) | 37 (5-109) |
Abbreviations: CNI=calcineurin inhibitor; CMV=Cytomegalovirus; Cy=cyclophosphamide; D-R=Donor-Recipient; Flu=fludarabine; Haplo=haploidentical; HCT-CI=hematopoietic cell transplant-comorbidity index; Mel=melphalan; MMF=mycophenolate mofetil; MSD=matched sibling donor; MTX=methotrexate; PTCy=post-transplantation cyclophosphamide; TBI=total body irradiation.
Patient race - other: MSD: 18 Asian; 12 Hispanic, race NOS; 2 Native Pacific Islander; 2 Native American unspecified; Race not reported: 4 USA; 1 UK; 2 France; 9 Saudi Arabia; 1 Sweden; 6 Australia; 1 Brazil; 3 Canada. Haplo: 6 Asian; 2 Hispanic, race NOS; 1 race NOS, Canada.
Details of conditioning regimens are given in Supplemental Table 2S
GVHD prophylaxis – haploidentical donor: 133 CNI + MMF + Cytoxan; 2 CNI + Cytoxan; 2 Cytoxan alone; 1 MMF + Cytoxan; 1 CNI + MTX+ Cytoxan.
Hematopoietic Recovery
The day 28 cumulative incidence of neutrophil recovery for the MSD/CNI platform patients was 98% (95%CI=96-99) compared 96% (95%CI=92-98) for the haplo/PTCy platform (P=0.25). The day 100 cumulative incidence of platelet recovery in the same order was 97% (95%CI=95-98) and 91% (95%CI=85-95) (P=0.04; Table 2), respectively. Median days from HCT to neutrophil and platelet recovery for MSD patients was 14 (3-132) and 17 (8-89) days compared to 17 (5-64) and 26 (11-103) days for haplo patients, respectively. (P=<0.001; Table 2).
Table 2.
Univariate outcomes.
| Matched sibling donor(N =457) |
Haploidentical donor (N =139) |
||||
|---|---|---|---|---|---|
| Outcomes | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | p-value |
| Neutrophil recovery | 449 | 137 | |||
| 28 days | 98 (96-99)% | 96 (92-98)% | 0.25 | ||
| Median time from HCT to neutrophil engraftment, days | 14 (3-132) | 17 (5-64) | <0.001 | ||
| Platelet recovery | 415 | 108 | |||
| 100 day | 97 (95-98)% | 91 (85-95)% | 0.04 | ||
| Median time from HCT to platelet recovery, days | 17 (8-89) | 26 (11-103) | <0.001 | ||
| Grade 2-4 acute GVHD | 447 | 136 | |||
| 180 days | 30 (26-35)% | 45 (37-53)% | 0.003 | ||
| Grade 3-4 acute GVHD | 420 | 127 | |||
| 180 days | 11 (8-14)% | 7 (3-12)% | 0.14 | ||
| Chronic GVHD | 444 | 133 | |||
| 1-year | 46 (41-51)% | 23 (16-31)% | <0.001 | ||
| 3-year | 56 (51-61)% | 28 (21-36)% | <0.001 | ||
| Extensive cGVHD | 434 | 132 | |||
| 1-year | 38 (34-43)% | 16 (10-23)% | <0.001 | ||
| 3-year | 45 (40-50)% | 18 (12-26)% | <0.001 | ||
| Non-relapse mortality | 457 | 139 | |||
| 1-year | 6 (4-8)% | 11 (6-17)% | 0.07 | ||
| 3-year | 10 (7-13)% | 14 (9-21)% | 0.19 | ||
| Relapse/progression | 457 | 139 | |||
| 1-year | 42 (37-46)% | 32 (24-40)% | 0.04 | ||
| 3-year | 56 (51-61)% | 48 (39-57)% | 0.14 | ||
| Progression-free survival | 457 | 139 | |||
| 1-year | 53 (48-57)% | 57 (49-65)% | 0.35 | ||
| 3-year | 34 (30-39)% | 38 (29-47)% | 0.53 | ||
| Overall survival | 457 | 139 | |||
| 1-year | 84 (80-87)% | 78 (71-85)% | 0.14 | ||
| 3-year | 63 (58-67)% | 63 (54-71)% | 0.99 | ||
Abbreviations: Eval=evaluable; GVHD=graft-versus-host disease; N=number; Prob=probability.
GVHD:
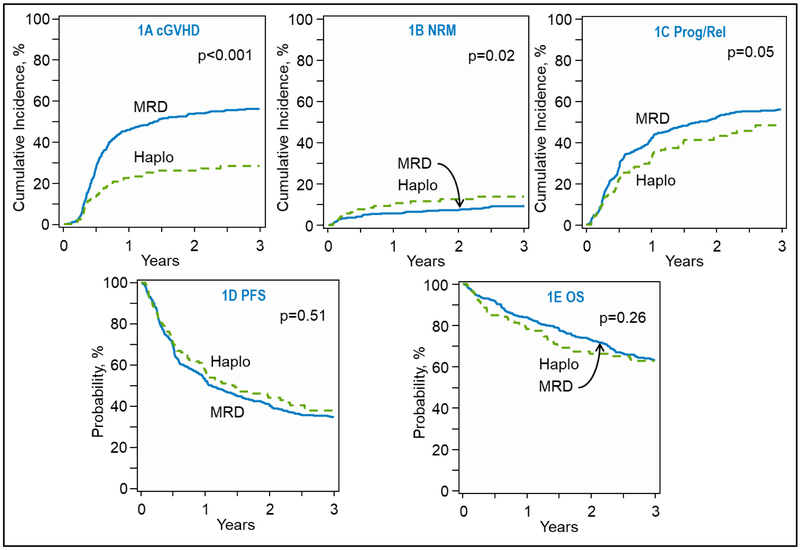
On univariable analysis the day 180 cumulative incidence of grade 2-4 acute GVHD after haplo/PTCy was higher at 45% (95%CI=37-53) compared 30% in MSD/CNI cohort (95%CI=26-35) (P=0.003; Table 2). The cumulative incidence of grade 3-4 acute GVHD was not significantly different between the two groups, 7% (95%CI=3-12) and 11% (95%CI=8-14) (P=0.14). Multivariable analysis showed that the risk of grade 2-4 acute GVHD after haplo/PTCy was significantly higher (OR=1.73; 95%CI=1.16-2.59; P=0.01; Table 3), however the risk of grade 3-4 acute GVHD was not significantly different between the two groups (OR=0.61; 95%CI=0.29-1.27; P=0.19) Other variables predictive of acute GVHD risk are shown in Table 3. The 1-year cumulative incidence of chronic GVHD was significantly higher in the MSD/CNI platform compared to the haplo/PTCy platform at 46% (95%CI=41-51) vs. 23% (95%CI=16-31) (P<0.001; Table 2 and Figure 1a). Accordingly extensive cGVHD was also higher in the MSD/CNI pairing compared to haplo/PTCy at both 1 and 3 years (Table 2). Multivariable analysis confirmed lower risk of chronic GVHD for the haplo/PTCy platform (HR=0.45; 95%CI=0.32-0.64; P<0.001; Table 3).
Table 3.
Multivariate analysis*
| N | HR | HR Lower CL | HR Upper CL | p-value | Overall p- value |
|
|---|---|---|---|---|---|---|
| Acute GVHD II-IV | ||||||
| Matched sibling donor | 447 | 1.00* | 0.01 | |||
| Haploidentical donor | 136 | 1.73* | 1.16 | 2.59 | 0.01 | |
| HCT-CI | ||||||
| 0 | 232 | 1.00 | 0.01 | |||
| 1-2 | 142 | 1.47 | 0.95 | 2.28 | 0.08 | |
| 3+ | 176 | 1.09 | 0.72 | 1.66 | 0.68 | |
| Missing | 33 | 0.14 | 0.03 | 0.61 | 0.01 | |
| Acute GVHD III-IV | ||||||
| Matched sibling donor | 420 | 1.00* | 0.19 | |||
| Haploidentical donor | 127 | 0.61* | 0.29 | 1.27 | ||
| Chronic GVHD | ||||||
| Matched sibling donor | 445 | 1.00 | <0.001 | |||
| Haploidentical donor | 133 | 0.45 | 0.32 | 0.64 | <0.001 | |
| KPS | ||||||
| ≥ 90% | 433 | 1.00 | 0.05 | |||
| <90% | 123 | 1.36 | 1.03 | 1.79 | 0.03 | |
| Missing | 22 | 0.70 | 0.34 | 1.41 | 0.32 | |
| Relapse | ||||||
| Matched sibling donor | 457 | 1.00 | 0.03 | |||
| Haploidentical donor | 139 | 0.74 | 0.56 | 0.97 | 0.03 | |
| KPS | ||||||
| ≥ 90% | 446 | 1.00 | 0.001 | |||
| <90% | 128 | 1.53 | 1.19 | 1.97 | 0.001 | |
| Missing | 22 | 1.08 | 0.61 | 1.89 | 0.80 | |
| Disease status | ||||||
| CR | 223 | 1.00 | <0.001 | |||
| PR | 260 | 2.06 | 1.58 | 2.69 | <0.001 | |
| Resistant | 100 | 2.77 | 2.01 | 3.82 | <0.001 | |
| Untreated/Missing | 13 | 2.34 | 1.08 | 5.07 | 0.03 | |
| Non-relapse mortality | ||||||
| Matched sibling donor | 457 | 1.00 | 0.06 | |||
| Haploidentical donor | 139 | 1.65 | 0.99 | 2.77 | 0.06 | |
| Age | ||||||
| 18-29 | 238 | 1.00 | 0.001 | |||
| 30-39 | 188 | 1.59 | 0.82 | 3.07 | 0.17 | |
| 40-49 | 94 | 2.13 | 1.01 | 4.49 | 0.05 | |
| ≥50 | 76 | 3.55 | 1.81 | 6.95 | <0.001 | |
| HCI-CI | ||||||
| 0 | 238 | 1.00 | 0.05 | |||
| 1-2 | 146 | 0.60 | 0.29 | 1.24 | 0.17 | |
| 3+ | 179 | 1.56 | 0.90 | 2.70 | 0.12 | |
| Missing | 33 | 1.49 | 0.51 | 4.31 | 0.47 | |
| Contrast | ||||||
| 1-2 vs. 3 | 0.39 | 0.19 | 0.78 | 0.01 | ||
| Progression-free survival | ||||||
| Matched sibling donor | 457 | 1.00 | 0.22 | |||
| Haploidentical donor | 139 | 0.86 | 0.68 | 1.10 | 0.22 | |
| KPS | ||||||
| ≥ 90% | 446 | 1.00 | <0.001 | |||
| <90% | 128 | 1.57 | 1.25 | 1.98 | <0.001 | |
| Missing | 22 | 0.93 | 0.54 | 1.60 | 0.80 | |
| Disease status | ||||||
| CR | 223 | 1.00 | <0.001 | |||
| PR | 260 | 1.83 | 1.45 | 2.32 | <0.001 | |
| Resistant | 100 | 2.49 | 1.87 | 3.32 | <0.001 | |
| Untreated/Missing | 13 | 2.35 | 1.19 | 4.66 | 0.01 | |
| Overall survival | ||||||
| Matched sibling donor | 457 | 1.00 | 0.66 | |||
| Haploidentical donor | 139 | 1.07 | 0.79 | 1.45 | 0.66 | |
| Age | ||||||
| 18-29 | 238 | 1.00 | 0.03 | |||
| 30-39 | 188 | 0.94 | 0.69 | 1.30 | 0.72 | |
| 40-49 | 94 | 1.23 | 0.85 | 1.78 | 0.27 | |
| ≥50 | 76 | 1.64 | 1.13 | 2.39 | 0.01 | |
| KPS | ||||||
| ≥ 90 | 446 | 1.00 | 0.001 | |||
| <90 | 128 | 1.63 | 1.22 | 2.17 | <0.001 | |
| Missing | 22 | 1.13 | 0.61 | 2.11 | 0.70 | |
| Disease status | ||||||
| CR | 223 | 1.00 | <0.001 | |||
| PR | 260 | 1.69 | 1.24 | 2.31 | <0.001 | |
| Resistant | 100 | 2.54 | 1.76 | 3.65 | <0.001 | |
| Untreated/Missing | 13 | 1.12 | 0.40 | 3.11 | 0.83 | |
Abbreviations: CL=confidence limit; CR=complete remission; HCT-CI=hematopoietic cell transplant-comorbidity index; HR=Hazard ratio; KPS=Karnofsky performance score; N=number; PR=partial remission.
Represent Odds ratio.
Fig 1.
(A) Cumulative incidence of chronic graft-versus-host-disease in recipients of sibling donor (MSD), and haploidentical donor (HAPLO) transplantations (overall, P, .<0.001). (B) Cumulative incidence of non-relapse mortality in recipients of MSD and HAPLO transplantations(overall, P , .02). (C) Cumulative incidence of relapse and/or progression in recipients of MSD and HAPLO transplantations (overall, P , .05). (D) Kaplan-Meier estimate of progression-free survival (PFS) in recipients of MSD and HAPLO transplantations (overall, P = .51). (E) Kaplan-Meier estimate of overall survival (OS) in recipients of MSD and HAPLO transplantations (overall, P = .26).
Non-relapse Mortality:
The cumulative incidence of NRM at 1-year in the haplo/PTCy group at 11% (95%CI=6-17) compared to 6% (95%CI=4-8) in the MSD/CNI group, (P=0.07) (Table 2 and Figure 1b). On multivariable analysis, there was a trend towards higher risk of NRM with haplo/PTCy approach (HR=1.65, 95%CI=0.99-2.77, P=0.06) (Table 3), however this did not attain statistical significance. Other variables independently associated with NRM risk were age ≥ 50 years (HR=3.55; 95%CI=1.81-6.95; P<0.001), and hematopoietic cell transplant-co-morbidity index (HCT-CI) of 1-2 relative to 3 (HR=0.39; P=0.01), (Table 3).
Relapse/Progression:
The 1-year cumulative incidence of relapse was significantly higher in MSD/CNI group at 42% (95%CI=37-46%) compared to 32% (95%CI=24-40%) in the haplo/PTCy group (P=0.04) (Table 2, Figure1c. In multivariable analysis, haplo-HCT was associated with a significantly reduced risk of relapse (HR=0.74, 95%CI=0.56-0.97, P=0.03) Additional factors predictive of relapse/progression risk included performance status and disease status as shown in Table 3.
Progression-free Survival:
PFS was not significantly different between the two groups on univariate analysis with 1-year and 3-year PFS in the MSD/CNI group being 53% (95%CI=48-57) and 34% (95%CI=30-39) respectively, while the haplo/PTCy group had 1-year and 3-year PFS of 57% (95%CI=49-65) and 38% (95%CI=29-47), respectively (Table 2, Figure 1d). Similarly, on multivariable analysis there was no significant difference between the two groups (Table 3). Factors impacting PFS were performance status and disease status at time of transplant as shown in Table 3.
Overall Survival:
The median follow-up for surviving patients was 37 months (range, 5-109 months) for haplo/PTCy cohort and 52 months (range, 2-101 months) for the MSD/CNI recipients. There was no significant difference in OS between the 2 platforms at either 1-year or 3-years. MSD/CNI OS at 1- and 3-years was 84% (95%CI=80-87) and 63% (95%CI=58-67), respectively while haplo/PTCy OS at 1- and 3-years was 78% (95%CI=71-85) and 63% (95%CI=54-71), respectively (Table 2, Figure 1e). These results were confirmed in multivariable analysis (Table 3). Other predictors of worse OS were age ≥50 years, poor performance status and cHL not being in complete remission at the time of allo-HCT, as shown in Table 3. Post-relapse survival (clock starting at relapse post HCT) for MSD/CNI patients versus haplo/PTCy was not significantly different (at 3-year 42% vs. 44%; p=0.78, respectively). No center effect was found for any outcomes.
Multivariable analysis with ATG/alemtuzumab patients excluded:
Sixty-six (14%) MSD cohort patients received ATG or alemtuzumab as part of their conditioning regimen. Since ATG/alemtuzumab administration can influence risk of GVHD, relapse and NRM, we repeated multivariate analysis after excluding these patients. As shown in Supplemental Table 3, results of this multivariate model were concordant with the overall study population (Table 3).
Subgroup analysis of patients receiving peripheral blood grafts:
Forty-two patients who had a haploidentical donor received a peripheral blood stem cell (PBSC) graft source and PTCy. When comparing their outcomes to the MSD/CNI cohort (receiving PBSC) there was no difference in the incidence of acute or chronic GVHD, or PFS. Similar to the overall analysis the NRM was higher in the haplo/PTCy group, while the 1-year cumulative incidence of relapse was also comparable to overall analysis with MSD/CNI group at 42% (95%CI=37-47%) compared to 26% (95%CI= 14-41%) in the haplo/PTCy group (P=0.03). Corresponding to the main analysis MSD/CNI OS at 3 years was 62% (95%CI=57-67), while haplo/PTCy was 49% (95%CI=30-69) with a p value of 0.23. Details of the subgroup analysis are listed in Table 4.
TABLE 4.
Subgroup analysis of patients receiving peripheral blood as graft type
| MSD (N = 439) |
Haplo (N = 42) |
||||
|---|---|---|---|---|---|
| Outcomes | N Eval |
Prob (95% CI) | N Eval | Prob (95% CI) | p-value |
| Grade 3-4 acute GVHD | 404 | 40 | 0.21 | ||
| 6 months | 11 (8-15)% | 5 (0-14)% | 0.09 | ||
| Chronic GVHD | 427 | 39 | 0.16 | ||
| 1-year | 46 (41-51)% | 35 (20-50)% | 0.15 | ||
| 2-year | 54 (49-59)% | 42 (26-59)% | 0.18 | ||
| 3-year | 56 (51-61)% | 42 (26-59)% | 0.11 | ||
| NRM | 439 | 42 | <0.001 | ||
| 1-year | 6 (4-8)% | 22 (11-35)% | 0.02 | ||
| 2-year | 8 (5-10)% | 22 (11-35)% | 0.03 | ||
| 3-year | 10 (7-13)% | 22 (11-35)% | 0.07 | ||
| Relapse/progression | 439 | 42 | 0.16 | ||
| 1-year | 42 (37-47)% | 26 (14-41)% | 0.03 | ||
| 2-year | 53 (48-58)% | 45 (28-62)% | 0.40 | ||
| 3-year | 57 (52-61)% | 45 (28-62)% | 0.21 | ||
| PFS | 439 | 42 | 0.51 | ||
| 1-year | 52 (47-57)% | 52 (37-67)% | 1.00 | ||
| 2-year | 40 (35-44)% | 33 (18-51)% | 0.48 | ||
| 3-year | 34 (29-39)% | 33 (18-51)% | 0.97 | ||
| Overall survival | 439 | 42 | 0.02 | ||
| 1-year | 84 (80-87)% | 68 (54-82)% | 0.04 | ||
| 2-year | 72 (68-77)% | 57 (40-73)% | 0.08 | ||
| 3-year | 62 (57-67)% | 49 (30-69)% | 0.23 | ||
Outcomes of transplantation according to remission status at HCT:
At the time of transplant 223 patients were in complete remission (CR), 260 were in a partial remission (PR) while 100 were deemed to have resistant disease. The NRM between these groups was not different however, not surprisingly those who were in a CR had a statistically significant lower rate of relapse and superior PFS and OS when compared to the PR and resistant disease groups. Details of relapse, PFS and OS based on remission status are to be found in Table 5.
TABLE 5.
Univariate outcomes by remission status at HCT
| CR (N = 223) | PR (N = 260) | Resistant (N = 100) |
|||||
|---|---|---|---|---|---|---|---|
| Outcomes | N Eval |
Prob (95% CI) |
N Eval |
Prob (95% CI) |
N Eval |
Prob (95% CI) |
p-value |
| NRM | 223 | 260 | 100 | 0.76 | |||
| 1-year | 7 (4-10)% | 6 (3-9)% | 9 (4-16)% | 0.58 | |||
| 2-year | 8 (5-12)% | 8 (5-12)% | 10 (5-17)% | 0.78 | |||
| 3-year | 12 (7-17)% | 10 (6-14)% | 10 (5-17)% | 0.86 | |||
| Relapse/progression | 223 | 260 | 100 | <0.001 | |||
| 1-year | 24 (18-30)% | 46 (40-52)% | 58 (48-67)% | <0.001 | |||
| 2-year | 33 (27-40)% | 57 (51-63)% | 66 (56-75)% | <0.001 | |||
| 3-year | 37 (31-44)% | 61 (55-67)% | 69 (60-78)% | <0.001 | |||
| PFS | 223 | 260 | 100 | <0.001 | |||
| 1-year | 70 (63-75)% | 48 (42-55)% | 33 (24-43)% | <0.001 | |||
| 2-year | 59 (52-66)% | 35 (29-41)% | 24 (16-33)% | <0.001 | |||
| 3-year | 51 (44-58)% | 29 (23-35)% | 20 (13-29)% | <0.001 | |||
| Overall survival | 223 | 260 | 100 | <0.001 | |||
| 1-year | 90 (85-93)% | 82 (77-86)% | 68 (59-77)% | <0.001 | |||
| 2-year | 83 (78-88)% | 68 (62-74)% | 56 (46-66)% | <0.001 | |||
| 3-year | 76 (69-82)% | 59 (52-65)% | 44 (33-54)% | <0.001 | |||
Causes of death:
Relapse was the leading cause of death for both groups, affecting 110 (58%) MSD/CNI-based recipients and 24 (41%) haplo/PTCy-based recipients. The next most common cause of death, after primary disease, in the haplo/PTCy group was infections, 20% compared to 9% in the MSD/CNI group. GVHD was the main cause of death in 6% of the MSD/CNI and 2% of the haplo/PTCy group. Detailed information about causes of death is shown in Supplemental Table 4.
DISCUSSION
Donor selection for allo-HCT is based upon many factors inclusive of donor availability, HLA-compatibility and importantly outcomes associated with transplant, specifically risk of severe GVHD, relapse, and NRM. In this large registry-based study, we analyzed specifically two distinct platforms of donor type and GVHD prophylaxis; MSD grafts with CNI-based prophylaxis compared to haplo grafts with PTCy-based prophylaxis. The main findings of our study are as follows: (1) OS and PFS were similar for the two platforms; (2) the risk of chronic GVHD was significantly lower in recipients of haplo/PTCy-based approaches; (3) there was lower incidence of relapse observed for patients who received haplo/PTCy-based platform and; 4) there was a non-significant trend towards higher NRM with haplo/PTCy-based allo-HCT, seemingly related to higher fatal infectious complications. This analysis demonstrates that haplo/PTCy alloHCT provides similar survival outcomes to MSD/CNI alloHCT across multiple centers with a meaningful decrease in the risk of chronic GVHD and relapse.
Presently there are several studies reporting the comparison of haplo HCT with conventional MSD or MUD HCT. Our study is the largest analysis to focus solely on the comparison of MSD/CNI platform vs. Haplo/PTCy-based approach, to ascertain the risk/benefit profile of a haplo graft relative to the historical gold-standard donor option (i.e. HLA-identical sibling).
The European Society for Blood and Marrow Transplantation (EBMT) published their registry analysis [5], comparing cHL patients who received PTCy–based haplo (n=98) HCT with outcomes of patients who received MSD (n=338) or MUD (n=273) HCT and reported a 1-year NRM of 13%, 21% and 17% after alloHCT in MSD, MUD and haplo transplants, respectively with a difference seen in the NRM risk between the MSD and MUD groups. Similarly Gauthier et al., published retrospectively comparing MSD (n=90) and haplo (n=61) recipients of NMA/RIC allo-HCT for cHL and reported a 2-year NRM of 9% and 12% for haplo and MSD HCT respectively.[19] Mariotti et al., compared outcomes between HLA-identical donors (HLAid) (MSD=29, MUD=5 – total 34) and haplo (n=30) donors for cHL patients who relapsed after an autologous transplant and 1-year NRM rate was 17%, with a tendency toward higher NRM after haplo relative to HLAid (26% versus 9% P=0.09).[2] Death was due mainly to cHL progression in the HLAid recipients (n=12 versus 4), whereas more patients died of complications after haplo-HCT (n=9 versus 6). In our study there is a trend towards higher NRM with haplo/PTCy possibly due to increased rate of fatal infections. Although no difference was observed in neutrophil engraftment between the two approaches, we do not have access to kinetics of immune reconstitution in the CIBMTR registry. There was also an increase in organ failure in the haplo/PTCy group (n=7; 12%) versus the MSD/CNI group (n=14; 7%). It is also important to highlight that the 1-year NRM of only 6% in MSD/CNI group in our study is lower than historically reported rates at 1-year (~10-15%) for such patients [5, 9-12], while the NRM rates of haplo/PTCy (1-year=11%), are consistent with recently published data [5]. Given the time period of the current study, it contain cHL patients included in the 2016 CIBMTR[11, 12] analyses of lymphoma patients who received either a haplo or MSD between the years of 2008-2013. However there is no overlap between patients who received in vivo T-cell depletion or the subsequent years of transplant.
Relapse was the primary cause of death after alloHCT for cHL patients in ours and other studies. In the retrospective study by Burroughs et al., 90 patients with cHL were treated with a NMA conditioning regimen followed by allo-HCT from MSD (n =38), MUD (n =24), or haplo (n =28) donors and relapse was lowest among haplo recipients.[13] Gauthier et al. did not find any difference in cumulative incidence of relapse between haplo and MSD groups. [19] In the current analysis, our findings reveal a lower relapse rate for patients receiving a haplo/PTCy-based HCT compared to MSD/CNI-based HCT, which is analogous with the findings of Mariotti et al., who also reported a 3-year cumulative incidence of disease relapse of 13% for haplo HCT versus 62% for HLAid HCT.[5] Globally, despite the curative possibility of alloHCT, the relapse rate remains disappointingly high; therefore, studies evaluating post transplant maintenance and consolidation are needed to address this important unmet need for this population.
Despite a higher risk of grade 2-4 acute GVHD with haplo/PTCy platform in our study, the risk of severe acute (grade 3-4) acute GVHD was not higher with this approach, while the risk of chronic GVHD was significantly lower (Table 3). The higher rate of grade 2 acute GVHD in haplo/PTCy cohort is line with results reported by EBMT recently and this mirrors other data supporting that PTCy appears effective at preventing grade 3-4 aGVHD but is associated with grade 2 aGVHD in a third to one half of patients [5]. A higher proportion of patients in the MSD cohort received ATG/alemtuzumab which could certainly generate the finding of decreased aGVHD, however it is interesting that in the separate MVA with ATG/alemtuzumab excluded, the MSD arm continues to have a lower rate of aGVHD. The higher risk of chronic GVHD for the MSD/CNI platform was seen on both univariate and multivariable analysis. GVHD-free relapse-free survival (GRFS) was an endpoint that we were not able to analyze given that ‘systemic immunosuppression requiring chronic GVHD’ (a defined event for GRFS) is not captured in our registry. Moreover, the striking difference in chronic GVHD seen across the two groups in our analysis (HR=0.45), essentially means that evaluation of GRFS will show a significant difference in similar direction and serve as a surrogate for chronic GVHD incidence difference. The distinct contrast between the rates of cGVHD could be related to the use of PTCy in the haplo group however we cannot discount that the graft source overwhelmingly was BM which has been proven to decrease the risk of cGVHD across disease states and conditioning regimens.[20] The subgroup analysis of patients receiving PBSC grafts would support the latter argument given that the differences in aGVHD and cGVHD are not seen, however the numbers are small and firm conclusions cannot be drawn. Recent data suggest that patients with both pre allo-HCT and post allo-HCT exposure to checkpoint inhibitors (CPI) may have an increased risk of acute GVHD.[21-23] Consensus guidelines by Herbaux et al., [24] highlight that there is no unanimity regarding optimal transplant strategy for patients previously treated with CPI, however there is agreement in that the goal should be to reduce the risk of GVHD and veno-occlusive disease. Furthermore, the recommendation is to preferentially use a BM graft and PTCy for GVHD prophylaxis for those who have received prior CPIs in an effort to decrease the risk of GVHD. In the CIBMTR registry, detailed information about pre-transplant treatments is available only for patients reported at the CRF level. Among the 80 CRF subject (MSD=47; haplo=33) in the current study, only 1 MSD and 5 haplo patients had prior CPI exposure. An ongoing prospective observational CIBMTR study will be evaluating the impact of prior CPI exposure in cHL patients undergoing allo-HCT.
Targeted immunotherapy approaches can achieve high rates of response in relapsed/refractory cHL, including after relapse from autologous HCT[25, 26]. In current practice, allo-HCT is generally reserved for cHL with both a prior autologous HCT and brentuximab vedotin (BV) failure. In such very high-risk patients, PD-1 blockade is also an important salvage option. While PD-1 inhibitors have undoubtedly shown remarkable activity in cHL, unfortunately in high risk subsets of patients failing both an autologous HCT and BV, the results of PD-1 blockade are modest with a median duration of response in the range of 7.8 – 11.9 months.[26, 27] This obviously is suboptimal for relapsed/refractory cHL patients (with median age in early 30s in most published data). Considering these data it is important to recognize that allo-HCT remains an integral option in the management and cure of relapsed cHL.
In this series we did not include myeloablative conditioning (MAC) due to the fact that only a small number of cHL patients in the CIBMTR registry received haplo grafts with MAC (n=19). This analysis has the limitations that are fundamentally associated with registry-based studies and although we performed a careful comparison adjusting for factors associated with transplant outcomes, there are likely to be differences that could affect our results that are not readily identifiable. The higher NRM in the haplo group may be associated with infections however we do not have data on immune reconstitution, a limitation of our analysis. A higher percentage of patients in the MSD/CNI group had a prior autologous transplant which may infer a more aggressive disease pattern, partially explaining the higher relapse rate in that group. The nature of data captured in the registry does not allow us to adequately assess pre-transplant salvage regimens, therapy for chronic GVHD and therefore the ability to quantify GRFS. Since most haplo/PTCy patients received BM grafts, we cannot speculate whether similar results could be expected if majority of haplo/PTCy recipients underwent a peripheral blood HCT. In conclusion, our findings suggest that the haplo/PTCy package provides survival outcomes comparable to MSD/CNI HCT, with an improvement in chronic GVHD rates and decrease in relapse risk.
Supplementary Material
Highlights.
Lower Incidence of Chronic GVHD in RIC Haploidentical Versus Matched Sibling Donor Transplantation for Hodgkin Lymphoma
Decreased Relapse in PTCy-based Haploidentical Versus Matched Sibling Donor Transplantation for Hodgkin Lymphoma
Acknowledgement:
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N00014-16-1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflict of interest: No disclosures to report.
REFERENCES
- 1.Martinez C, Canals C, Sarina B et al. Identification of prognostic factors predicting outcome in Hodgkin's lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol 2013; 24: 2430–2434. [DOI] [PubMed] [Google Scholar]
- 2.Mariotti J, Devillier R, Bramanti S et al. T Cell-Replete Haploidentical Transplantation with Post-Transplantation Cyclophosphamide for Hodgkin Lymphoma Relapsed after Autologous Transplantation: Reduced Incidence of Relapse and of Chronic Graft-versus-Host Disease Compared with HLA-Identical Related Donors. Biol Blood Marrow Transplant 2018; 24: 627–632. [DOI] [PubMed] [Google Scholar]
- 3.Gajewski JL, Phillips GL, Sobocinski KA et al. Bone marrow transplants from HLA-identical siblings in advanced Hodgkin's disease. J Clin Oncol 1996; 14: 572–578. [DOI] [PubMed] [Google Scholar]
- 4.Milpied N, Fielding AK, Pearce RM et al. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin's disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol 1996; 14: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 5.Martinez C, Gayoso J, Canals C et al. Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation as Alternative to Matched Sibling or Unrelated Donor Transplantation for Hodgkin Lymphoma: A Registry Study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol 2017; 35: 3425–3432. [DOI] [PubMed] [Google Scholar]
- 6.Tomblyn M, Brunstein C, Burns LJ et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2008; 14: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hale GA, Shrestha S, Le-Rademacher J et al. Alternate donor hematopoietic cell transplantation (HCT) in non-Hodgkin lymphoma using lower intensity conditioning: a report from the CIBMTR. Biol Blood Marrow Transplant 2012; 18: 1036–1043 e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sureda A, Robinson S, Canals C et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2008; 26: 455–462. [DOI] [PubMed] [Google Scholar]
- 9.Raiola A, Dominietto A, Varaldo R et al. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin's lymphoma. Bone Marrow Transplant 2014; 49: 190–194. [DOI] [PubMed] [Google Scholar]
- 10.Castagna L, Bramanti S, Devillier R et al. Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone Marrow Transplant 2017; 52: 683–688. [DOI] [PubMed] [Google Scholar]
- 11.Kanate AS, Mussetti A, Kharfan-Dabaja MA et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 2016; 127: 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh N, Karmali R, Rocha V et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. J Clin Oncol 2016; 34: 3141–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burroughs LM, O'Donnell PV, Sandmaier BM et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2008; 14: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacigalupo A, Ballen K, Rizzo D et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P et al. 1994 Consensus Conference on Acute GVHD Grading Bone Marrow Transplant 1995; 15: 825–828. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217. [DOI] [PubMed] [Google Scholar]
- 18.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal 1995; 1: 145–156; discussion 157-149. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier J, Poire X, Gac AC et al. Better outcome with haploidentical over HLA-matched related donors in patients with Hodgkin's lymphoma undergoing allogeneic haematopoietic cell transplantation-a study by the Francophone Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transplant 2018; 53: 400–409. [DOI] [PubMed] [Google Scholar]
- 20.Bashey A, Zhang MJ, McCurdy SR et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 2017; 35: 3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv 2017; 1: 2643–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haverkos BM, Abbott D, Hamadani M et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood 2017; 130: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merryman RW, Kim HT, Zinzani PL et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017; 129: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbaux C, Merryman R, Devine S et al. Recommendations for managing PD-1 blockade in the context of allogeneic HCT in Hodgkin lymphoma: taming a necessary evil. Blood 2018; 132: 9–16. [DOI] [PubMed] [Google Scholar]
- 25.Younes A, Gopal AK, Smith SE et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 2012; 30: 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armand P, Engert A, Younes A et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol 2018; 36: 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016; 17: 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.