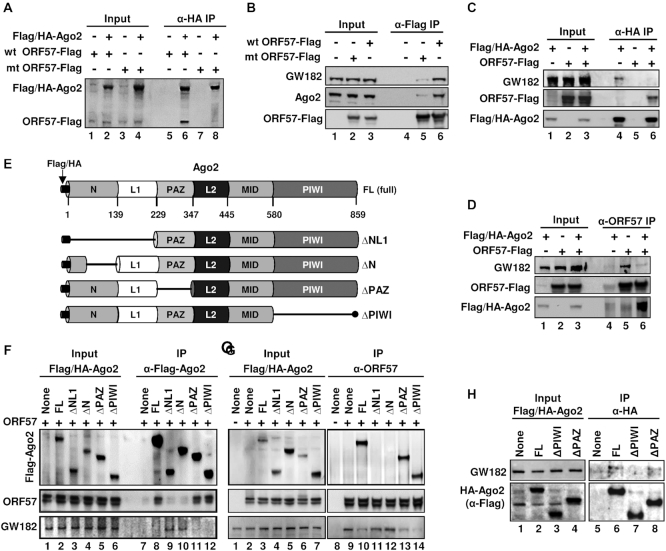
Figure 6.
Viral ORF57 interacts with GW182 and Ago2 and affects their binding activity. (A and B) ORF57-wt, but not its dysfunctional mutant (ORF57-mt), interacts with Ago2 and GW182. HEK293T cells were transfected separately with an empty vector (-) or a vector expressing Flag-tagged ORF57-wt or ORF57-mt or Flag/HA-tagged Ago2. Total cell lysate at 24 h after each transfection was mixed as indicated and digested with RNase A/T1 before co-immunoprecipitation for ORF57-associated proteins using an anti-HA antibody (A) or the cell lysates from transfection of an empty vector, Flag-ORF57-wt or Flag-ORF57-mt were digested with RNase A/T1 and co-immunoprecipitated for ORF57-associated endogenous GW182 and Ago2 using an anti-Flag antibody (B). The proteins in the co-IP were blotted for Ago2 and ORF57 by an anti-Flag antibody (A) or for GW182, Ago2 and ORF57 by the corresponding antibodies (B). (C and D) ORF57 disrupts Ago2–GW182 interaction. Total cell extract from HEK293T cells transfected by a vector expressing Flag/HA-Ago2, ORF57-Flag or an empty vector was mixed each other as indicated and then digested with RNase A/T1. The resulting protein complexes were co-immunoprecipitated by anti-HA (C) or anti-ORF57 (D) antibody-immobilized beads. The proteins in the co-IP were blotted for GW182, Flag/HA-Ago2 and ORF57 by the corresponding antibodies.(E–G) ORF57 interacts with the N-terminal domain of Ago2 and prevents Ago2 interaction with GW182. (E) Schematic diagrams (not in scale) of full-length (FL) Ago2 and its deletion mutants with the deleted region indicated by a solid line shows six characteristic domains of Ago2 (N, L1, PAZ, L2, MID and PIWI). (F and G) Mapping of ORF57-Ago2 interacting domains and their effect on Ago2–GW182 binding. HEK293T cell extract containing HA/Flag-Ago2 or its deletion mutant ΔNL1, ΔN, ΔPAZ or ΔPIWI was separately mixed with the cell extract containing untagged ORF57. The individual mixture was then digested with RNase A/T1 and immunoprecipitated using anti-Flag antibody for Ago2 (F) or anti-ORF57 antibody (G). The proteins in the co-IP were blotted for Ago2-associated ORF57 and endogenous GW182 (F) or ORF57-associated Flag/HA-Ago2 and endogenous GW182 (G) with the corresponding antibodies. A smaller band of ORF57 is the caspase-7 cleaved ORF57 (82). (H) The PIWI domain, not PAZ, of Ago2 is required to interact with GW182. HEK293T cell extract containing Flag/HA-Ago2 or its deletion mutant ΔPAZ or ΔPIWI were digested with RNase A/T1 and immunoprecipitated overnight using anti-HA-coated beads. The immunoprecipitated complexes were eluted by HA peptides and analyzed by Western blot using an anti-Flag antibody to detect Ago2 and anti-GW182 human serum to detect the endogenous GW182.