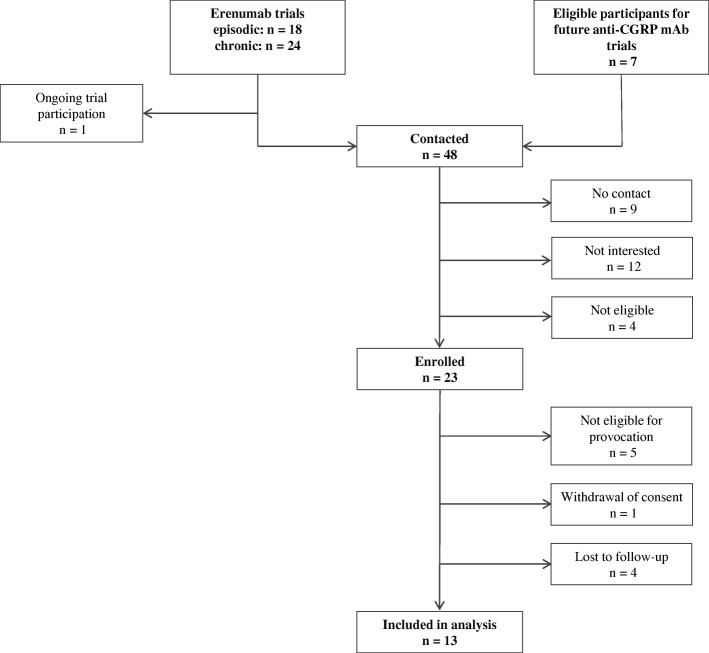
Fig. 3.
Inclusion flowchart. Twenty-three patients were enrolled in the study. Ten of these were excluded subsequently. One patient was excluded due to a cardiac conduction disease and one due to diabetes mellitus (well-regulated), according to the conventional CGRP provocation protocol. Three patients were excluded from analysis as they did not participate in the erenumab trials. One patient withdrew consent before the experiments. Four patients were lost to follow-up and one of these had participated in the first study day. Data from these days were excluded from analyses. Of the ten patients, who were excluded, seven had received erenumab and six of these were high responders. Response status was not obtained from the last of the seven subjects