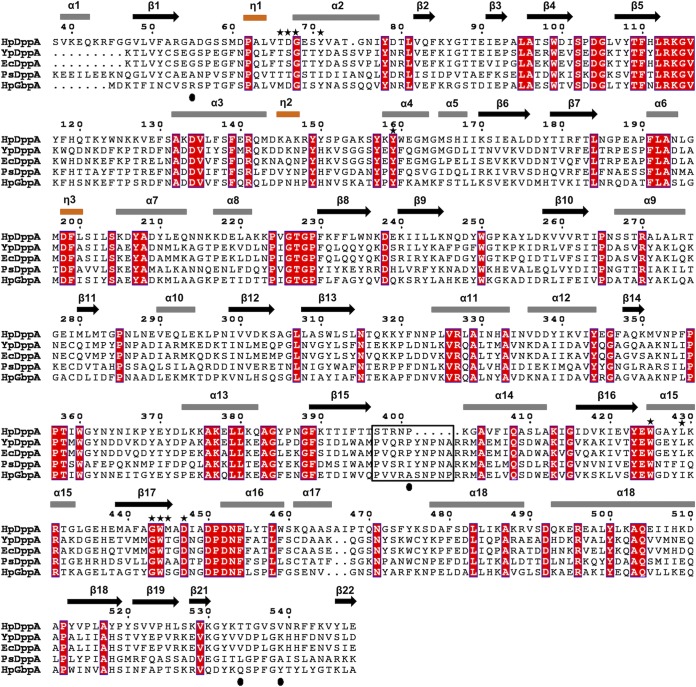
FIG 7.
A structure-based sequence alignment of HpDppA with DppAs and a glutathione-binding protein. HpDppA, DppA from H. pylori SS1; YpDppA, DppA from Y. pestis; EcDppA, DppA from E. coli; PsDppA, DppA from Pseudoalteromonas sp. SM9913; HpGbpA, glutathione-binding protein GbpA from H. parasuis. The secondary structure elements and the sequence numbering for HpDppA are displayed above the alignment. Conserved residues are boxed in red. The residues of HpDppA that form direct and water-mediated interactions with the bound tetrapeptide are marked with stars. The unique ligand-binding-site residues that are present in HpGbpA but absent in DppAs are indicated by filled circles. Residues that form the loop from position 397 to 401 in HpDppA and the equivalent loop residues in other proteins are highlighted using a black box. The image was generated using ESPript (http://espript.ibcp.fr).