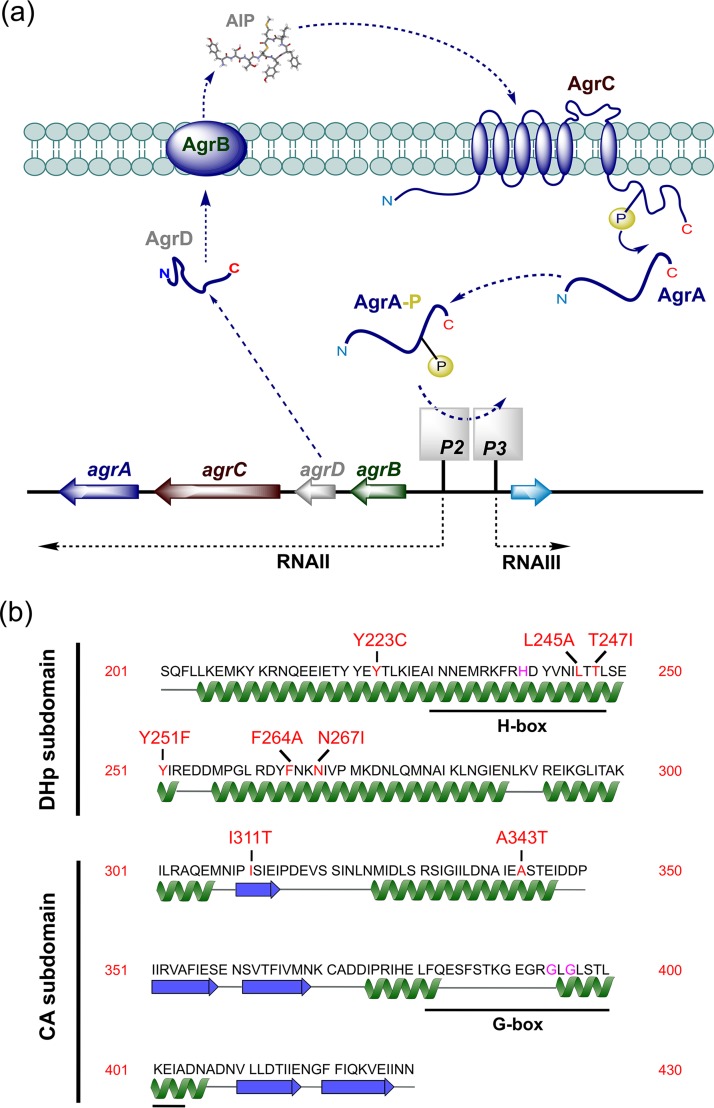
FIG 1.
(a) Schematic of the agr-dependent quorum-sensing system. Two divergent transcripts (RNAII and RNAIII) are driven by the agrP2 and agrP3 promoters, respectively. The agrBDCA operon is in RNAII. The autoinducing peptide (AIP) is generated from the AgrD peptide precursor via AgrB. The sensor kinase, AgrC, autophosphorylates on binding AIP, resulting in phosphorylation of the response regulator AgrA to activate the P2 and P3 promoters. Downstream target genes are activated by AgrA either directly or indirectly via RNAIII, which also codes for delta-hemolysin. (b) Secondary structure of the cytoplasmic domain of AgrC indicating the location of the dimerization and histidine phosphorylation (DHp) and catalytic and ATP-binding (CA) subdomains. The key functional and mutated residues are marked in purple and red, respectively. The H-box histidine (His239) is the predicted phosphorylation site, and G-box glycine residues (Gly394 and Gly396) are critical for ATP binding. Functional annotations were adapted from Cisar et al. (37).