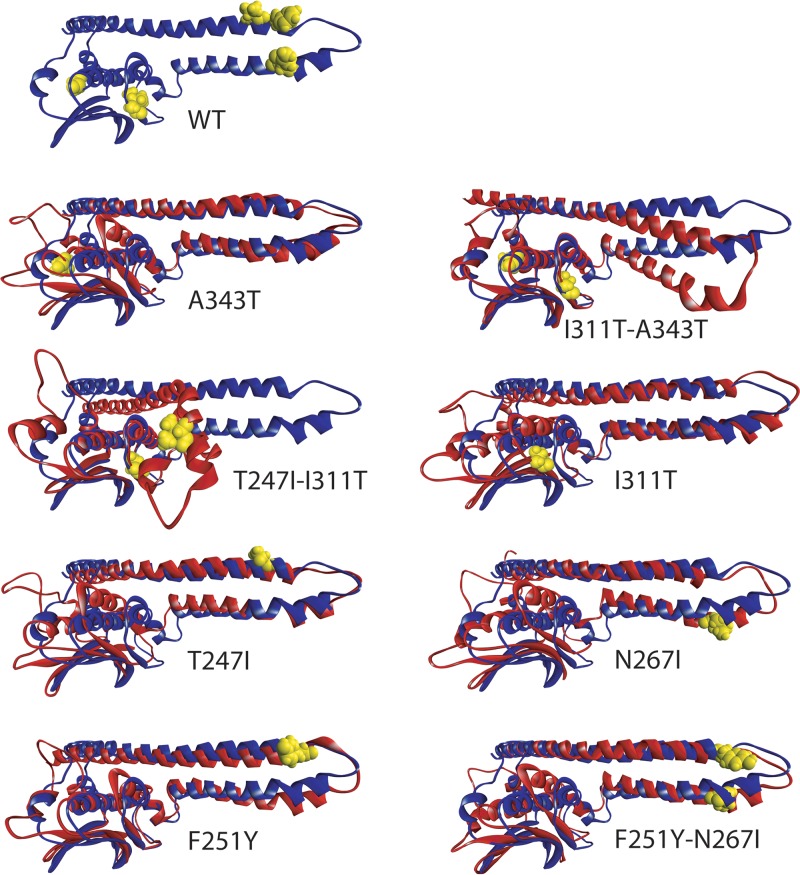
FIG 6.
MD-annealed conformation of wild type (WT) AgrC (blue). Side chains are shown in yellow in the WT structure for all mutated residues. Single and double point mutants have been annealed according to an identical protocol and starting structure as for the WT; end structures (red) are shown aligned pairwise to the annealed WT structure (blue). In each pair, mutated residues are shown in full atom representation in yellow for each AgrC substitution shown. Subtle changes near the turn of the helical hairpin translate to markedly reduced accessibility of the ATP-binding pocket (see Fig. 7). Since the WT AgrC already contained a Y251F substitution, this is reversed as F251Y for comparison. In the double mutant T247I-I311T, the helical hairpin buckles and collapses over the CA subdomain, restricting access to the putative AgrA-binding site near the helical turn.