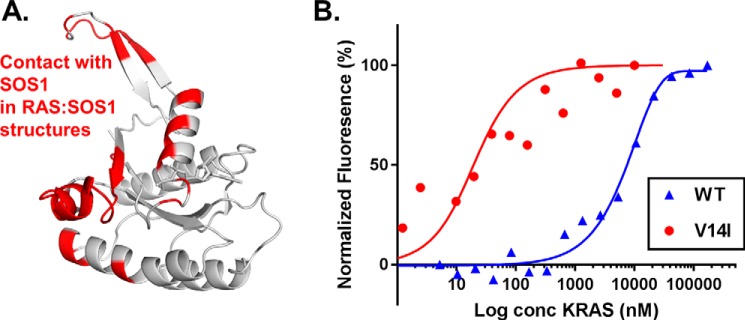
Figure 5.
Interaction between KRASV14I and SOS1. A, the binding interface of KRASV14I for SOS1 based on PDB 1NVU. Interacting residues on KRASV14I are highlighted in red. B, SOS1:KRAS binding affinity as measured by microscale thermophoresis. KRASV14I shows enhanced affinity toward SOS1 relative to KRASWT. All measurements were performed in triplicate: Kd for WT is 8.3 ± 0.6 (μm) and V14I is 0.22 ± 0.1 (μm). The SOS1 construct includes the REM and catalytic domains.