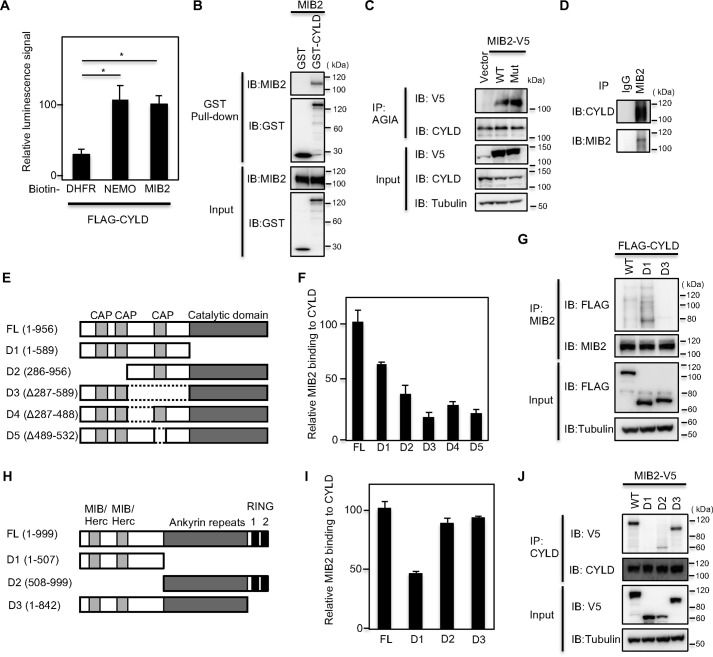
Figure 1.
MIB2 interacts with CYLD. A, determination of CYLD-MIB2 interaction by AlphaScreen. FLAG-CYLD and biotinylated DHFR, NEMO, or MIB2 were synthesized by using a wheat cell–free system, and their interactions were analyzed by AlphaScreen. Mean ± S.E. (n = 4). Statistical significance was assessed using one-way ANOVA. *, p < 0.01. B, determination of CYLD-MIB2 interaction by pulldown assay. GST pulldown assay was carried out using control GST or GST-CYLD fusion proteins on Sepharose beads followed by incubation with MIB2. C, analysis of CYLD-MIB2 interaction in cells. Immunoprecipitation using an anti-AGIA antibody was performed from extracts of HEK293T cells transfected with V5-tagged MIB2 and AGIA-tagged CYLD. The presence of MIB2 in the immunoprecipitate was evaluated by immunoblotting with the respective antibody. The MIB2 mutant (Mut) used contained a CS mutation in the two RING domains. D, determination of endogenous CYLD-MIB2 interaction. Immunoprecipitation using either control IgG or anti-MIB2 antibody was performed from HEK293T cell extracts. The endogenous interaction of MIB2 with CYLD was evaluated by immunoblotting with an anti-CYLD antibody. E, schematic representation of full-length CYLD (FL), along with its various deletion mutants (D1–D5). F, identification of CYLD-binding region in vitro. The AlphaScreen analysis was performed between MIB2 and CYLD or its deletion mutants. Biotinylated MIB2, or FLAG-CYLD FL and its various deletion mutants, were synthesized by using wheat cell–free system, and then were used. G, identification of CYLD-binding region in cell. HEK293T cells expressing endogenous MIB2 were transfected with the indicated FLAG-tagged CYLD constructs and the interaction between CYLD and MIB2 was determined by immunoprecipitation and immunoblotting with the indicated antibodies. H, schematic representation of full-length MIB2 (FL), along with its various deletion mutants (D1–D3). I, identification of MIB2-binding region in vitro. The AlphaScreen signals between CYLD and MIB2 or its deletion mutants. Biotinylated CYLD or FLAG-MIB2 FL and its various deletion mutants were used. J, identification of MIB2-binding region in cell. HEK293T cells expressing endogenous CYLD were transfected with the indicated V5-tagged MIB2 constructs, and the interaction between CYLD and MIB2 was determined by immunoprecipitation and immunoblotting with the indicated antibodies.