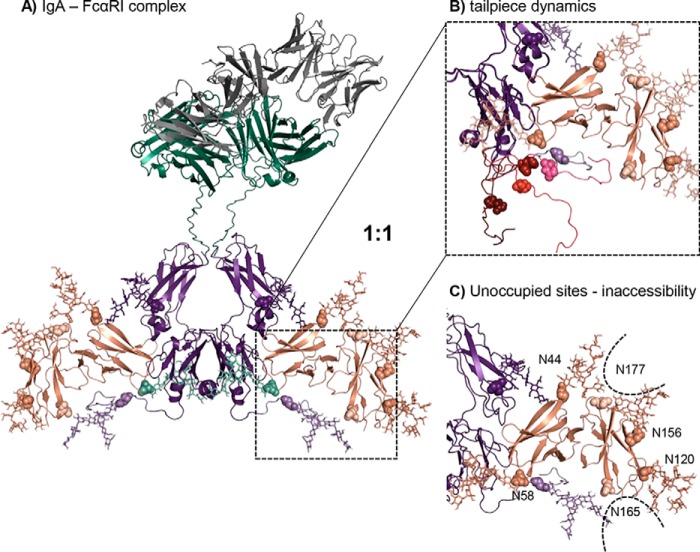
Figure 7.
The molecular model of N-glycosylated IgA1-Fc in complex with FcαRI suggests a 1:1 binding stoichiometry. A, IgA1-Fc region colored in purple, constant heavy and light chains colored in green, variable region colored in gray, and FcαRI colored in salmon. IgA1-Fc has a CH2-resident and a tailpiece N-glycan (shown in lighter purple; N-glycosylation sites depicted in spheres and N-glycans depicted as sticks). FcαRI has six potential N-glycosylation sites at Asn-44, Asn-58, Asn-120, Asn-156, Asn-165, and Asn-177 shown in spheres. The two N-glycosylation sites Asn-165 and Asn-177, which are hardly or not occupied, are colored in a lighter color. Complex (GnGn) N-glycans of IgA1-Fc and oligomannosidic (Man9) N-glycans of FcαRI are shown as sticks. B, five different tailpiece conformations (each shown in a different color) were aligned to the model where the backbone is shown as a cartoon, the tailpiece N-glycosylation site is marked in spheres, and glycans are shown as sticks. C, N-glycosylation sites Asn-165 and Asn-177 show the lowest SASA with ∼67 Å2, rendering the site inaccessible for N-glycosylation.