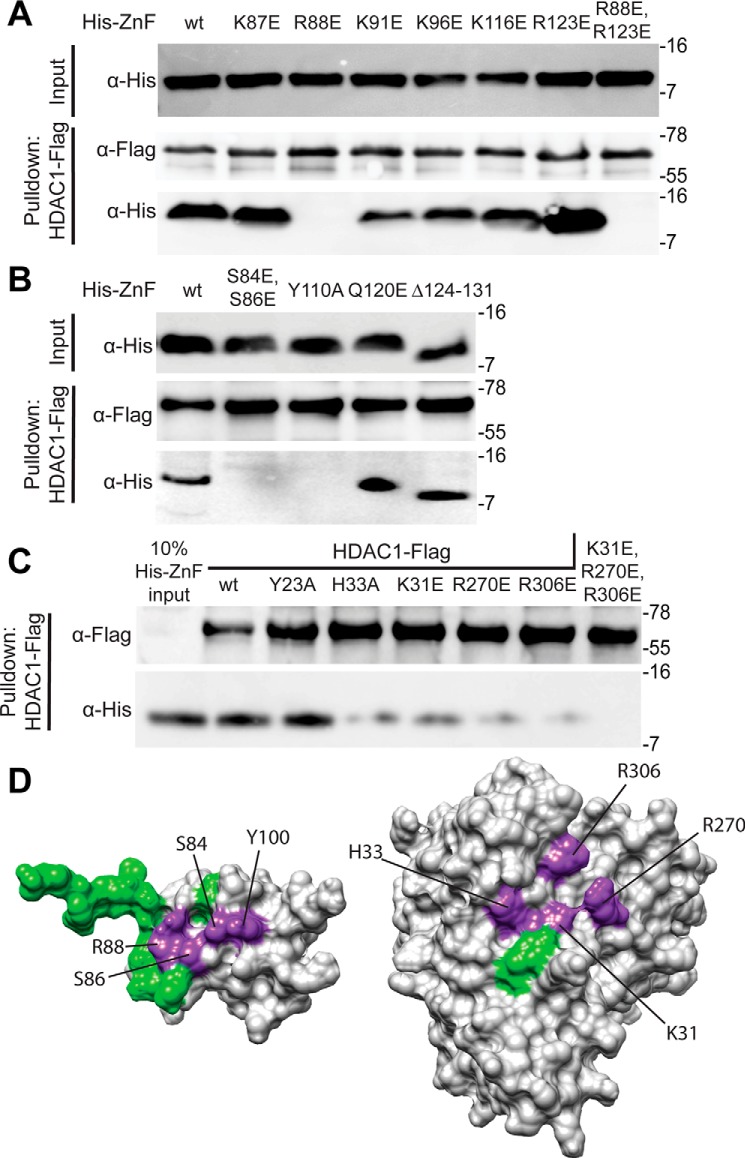
Figure 2.
Mapping residues at the interfaces of the SAP30 ZnF–InsP6–HDAC1 complex via mutagenesis and interaction assays. A and B, pulldown assays conducted with immobilized FLAG-tagged HDAC1 and His6-tagged SAP30 ZnF single-site mutants of candidate basic residues involved in engaging with inositol phosphates (A) or other solvent-exposed conserved residues (B). The protein labeled Δ124–131 corresponds to SAP30 ZnF, which has a C-terminal deletion; the construct spans residues 64–123. C, pulldown assays with immobilized FLAG-tagged WT or mutant HDAC1 and His6-tagged SAP30 ZnF. All pulldown experiments were conducted in the presence of InsP6. D, mutations mapped onto the surface of SAP30 ZnF (right) and HDAC1 (left). Purple indicates mutations that lead to loss of binding in pulldown experiments, and green indicates that no change was observed. In A–C, molecular mass (in kilodaltons) is shown on the right of each blot. AU, arbitrary units.