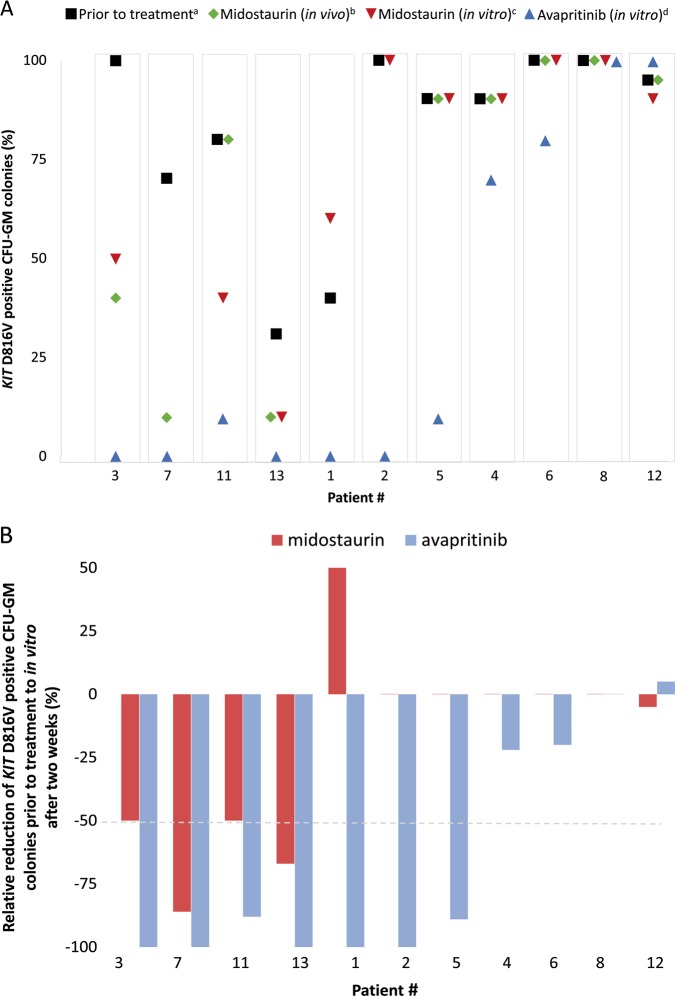
Fig. 2.
a Summarizes in vivo and in vitro data regarding the proportion of KIT D816V positive single-cell-derived myeloid progenitor cells (CFU-GM colonies) for each patient: aprior to treatment, bcolonies after six months midostaurin-treatment in vivo, ccolonies incubated in vitro with midostaurin for two weeks, dcolonies incubated in vitro with avapritinib for two weeks. CFU-GM, granulocyte-macrophage colony-forming-unit. b Relative reduction in the proportion of KIT D816V positive colonies from baseline (prior to treatment) to in vitro colonies incubated with midostaurin (red) and avapritinib (blue). In patient #7, midostaurin in vivo data was used (in vitro data not available). Patient order is based on response pattern (responder: at least 50% relative reduction of KIT D816V positive colonies): midostaurin + avapritinib responder (cohort #1; patient #3, #7, #11, #13), midostaurin non-responder + avapritinib-responder (cohort #2; patient #1, #2, #5), and midostaurin + avapritinib non-responder (cohort #3; patient #4, #6, #8, #12). CFU-GM granulocyte-macrophage colony-forming-unit