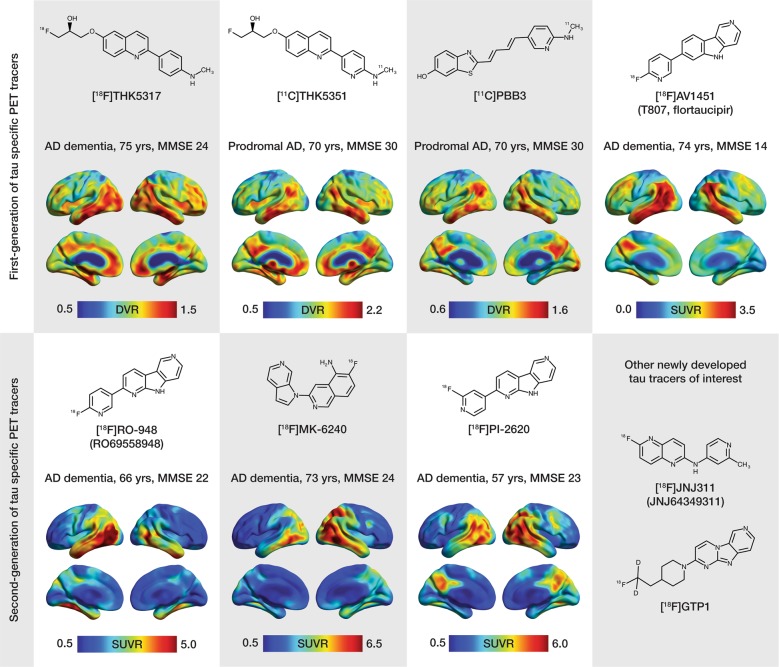
Fig. 2.
Chemical structures and representative uptake images in amyloid-β-positive Alzheimer’s disease (AD) patients using selected first- (upper portion of the figure) and second- (lower portion of the figure) generation tau PET tracers. The characteristics in terms of clinical research diagnosis, age and mini mental-state examination (MMSE) scores are presented for each patient above the respective image. For the creation of parametric images for all tracers, areas of the cerebellar cortex were used as reference. The [18F]THK5317, [11C]THK5351 and [11C]PBB3 images derive from studies performed at Karolinska Institutet, Center for Alzheimer Research [35, 153]. The [18F]AV1451 image is courtesy of the Alzheimer’s disease neuroimaging initiative (ADNI). The [18F]RO-948 image is courtesy of Ruben Smith and Oskar Hansson (Lund University, Lund, Sweden). The [18F]MK-6240 and the [18F]PI-2620 images are courtesy of Vincent Doré, Christopher Rowe and Victor Villemagne (University of Melbourne, Victoria, Australia) and Andrew Stephens and Mathias Berndt (Piramal Imaging GmbH, Berlin, Germany), respectively. Different scales were used to better illustrate the regional distribution pattern of binding for each tracer, due to between-patient differences as well as due to the different PET acquisition parameters and quantification methods that were applied for each tracer. Though direct comparison is complicated by these differences, one can observe the preferential binding of the first- and second-generation tracers in AD-relevant areas of the temporal lobes, and the broader dynamic range among second-generation tracers. DVR distribution volume ratio, SUVR standardized uptake value ratio