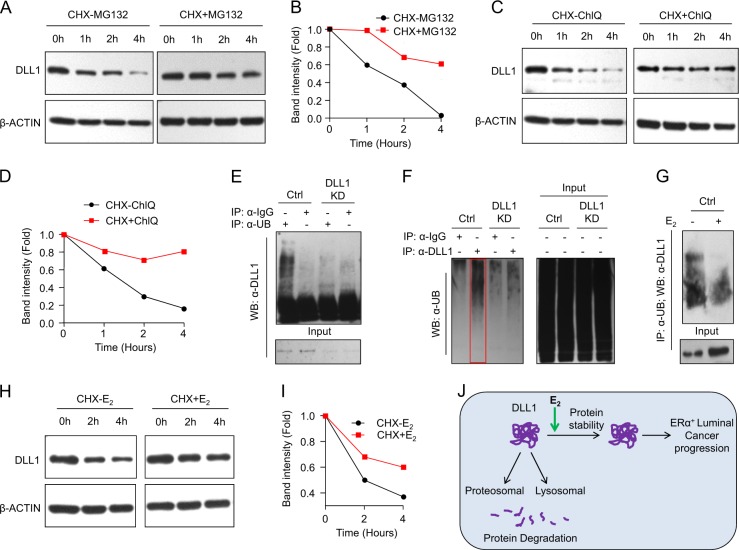
Fig. 8.
E2/ERα signaling stabilizes DLL1 protein levels from proteosomal and lysosomal degradation. a, b Western blot (a) shows decreased DLL1 protein levels in MCF7 cells after blocking of protein synthesis using cyclohexamide (CHX) at indicated timepoints, which is reversed with the treatment of MG132, a proteosomal inhibitor. Quantification of bands was done using ImageJ and is shown in (b). c, d Western blot shows decreased DLL1 protein levels in MCF7 cells after blocking of protein synthesis using cyclohexamide (CHX) at indicated timepoints, which is reversed with the treatment of Chloroquinone (ChlQ), a lysosomal inhibitor. Quantification of bands was done using ImageJ and is shown in (d). e, f Western blot shows polyubiquitination of endogenous DLL1 protein in hormone-deprived MCF7 cells. MCF7 (control and DLL1 KD1) cells were subjected to endogenous immune-precipitation (IP) with anti-Ubiquitin or anti-DLL1 antibodies followed with western blot using either anti-DLL1 or anti-Ubiquitin-specific antibodies respectively. Respective IgG controls were used as a negative control for IP. g Western blot shows decreased polyubiquitination of endogenous DLL1 protein upon 6 h of E2 treatment to MCF7 cells. MCF7 cells were subjected to endogenous immune-precipitation (IP) with anti-Ubiquitin antibody followed with western blot using anti-DLL1 antibody. h, i Western blot (h) and quantification (i) of DLL1 protein levels after blocking of protein synthesis using cyclohexamide (CHX) with or without E2 treatment at indicated timepoints in MCF7 cells. Quantification of bands was done using ImageJ software. j Schematic showing DLL1 protein degradation happens through proteosomal and lysosomal degradation, which is prevented by Estrogen signaling through E2/ERα in luminal breast cancer for promotion and progression of the luminal breast cancer