Abstract
Objective
This study aimed to assess the effect of carbon dioxide (CO2) laser on prevention of white spot lesions (WSLs) associated with fixed orthodontic treatment.
Methods
In this parallel controlled trial, 554 maxillary anterior teeth in 95 patients with age range of 12–30 years were included. The samples were randomly divided in two groups: 1) CO2 laser (n=278) and 2) control (n=276) groups. Following bracket attachment, the teeth in the laser group were exposed to CO2 laser (0.4 mw, 10.6 μm, 5 Hz) for 20 s, and the control group received placebo light. Incidence, severity, and extent of the lesions were assessed in four surface regions (gingival, incisal, mesial, and distal) at baseline and 6 months post-irradiation. The inter-group comparison was performed by the Mann-Whitney U test and McNemar analysis.
Results
A significant difference regarding WSLs incidence in all teeth was observed between the two study groups (p<0.001). The two study groups illustrated a significant difference in lesion extent and incidence in incisal, mesial, and distal regions (p<0.05). The WSLs were significantly different in terms of severity in the incisal and mesial sites (p<0.05).
Conclusion
The CO2 laser irradiation seemed to effectively prevent incidence of WSLs. In addition, its effectiveness varied depending on the surface region.
Keywords: Carbon dioxide laser, clinical trial, orthodontic treatment, white spot lesion
INTRODUCTION
One of the most common side effects of fixed orthodontic appliances is white spot lesions (WSLs) around the orthodontic bands and brackets (1). Fixed brackets increase the number of susceptible sites to plaque accumulations, and they disturb the balance between enamel demineralization and remineralization processes. This phenomenon ultimately leads to mineral loss and development of WSLs (2). WSLs are enamel subsurface porosities with an opaque milky-white appearance. Previous studies estimated the incidence of these lesions in the fixed orthodontic treatments ranging from 50%–70% (2, 3). Øgaard et al. (4) demonstrated that prevalence of this condition is significantly higher in orthodontic patients even five years post-treatment. Thus, prevention of WSLs is crucial to inhibit the smile esthetics from being compromised.
One method to increase caries resistance is laser irradiation. CO2 laser is one of the most popular and efficient sources of coherent electromagnetic waves in the infrared spectrum introduced by Patel et al. (5) in 1964. Several studies have suggested that CO2 laser is most effective in prevention of caries, whilst some researches assumed that it was more effective on the soft tissues (6–8). Rodrigues et al. (7) showed that CO2 laser irradiation increased the acid resistance of enamel due to change in the hydroxyapatite crystals. The CO2 laser irradiation is assumed to coincide with the absorption bands of carbonate, phosphate, and hydroxyl groups in the enamel and dentin structure. Accordingly, temperatures increasing at the enamel surface and subsurface result in the chemical and structural alterations, such as carbonate content reduction, decomposition of organic matrix, crystals formation of hydroxyapatite, and finally more resistance to acidic attacks (9). Also, Esteves-Oliveira et al. (6) observed less mineral loss and the re-hardening of softened enamel in the samples treated only by CO2 laser; however, the combination of fluoride and subsequent CO2 laser irradiation was effective on the inhibition of surface microhardness change. Moreover, they explained that the crystal growth related to the temperature variations, bigger crystals, and less crystallographic imperfections could be the reason for this improvement in hardness. In 2017, Paulos et al. (8) conducted a research on 65 human teeth to study the effect of CO2 and Nd:YAG laser alone and in combination with fluoride on prevention of enamel caries after periodic acidic challenges. Their findings showed that CO2 laser alone (with a wavelength of 10.6 μm) prevented enamel demineralization around the brackets even after repeated acidic challenges, and therefore had a deeper effect. In addition, Ramalho et al. (10) concluded that when compared to the fluoride group in all storage periods, both CO2 laser irradiation alone and the combined fluoride-laser treatment caused less mineral loss. Laser and fluoride have synergistic effect, and they improve the acid resistance of enamel that may be due to the organic matrix removal, enhanced fluoride uptake, and larger surface area for ions binding, including calcium and fluoride. Fluoride changes the bacterial plaque, alters demineralization and remineralization process, and induces calcium fluoride deposition and formation of the fluorohydroxyapatite crystals. These effects depend on the retention of the reaction products over time. Because the several times of topical fluoride application are essential to maintain the anti-caries effect, lasers are alternatively used to prevent caries because of the strong interaction with dental hard tissues (11).
Many studies have been conducted on the effect of CO2 laser on caries prevention or microhardness enhancement in laboratory conditions (6, 7). Because the scarce numbers of the clinical studies have been focused on this topic, this study aimed to assess the effect of CO2 laser on the prevention, severity, and the extent of WSLs in clinical conditions. Our hypothesis was that CO2 laser irradiation has preventive effect on WSLs during fixed orthodontic treatment.
METHODS
This double blind controlled clinical trial began April 2017 in Department of Orthodontics at Hamadan School of Dentistry, Iran. This research was approved by the ethics committee with IR.UMSHA.REC.1396.146 code and registered at www.irct.ir with IRCT2017052927362N2 identifier. The eligible patients were recruited from the Department of Orthodontics at Hamadan Dental School and an orthodontic clinic. The patients were included if they were 12–30 years old with maxillary anterior teeth and required orthodontic treatment. Because the number of patients referred to the department and clinic was low, the age range was considered wide. Also, medical and dental history, intraoral clinical and radiographic examinations were performed. The patients had to accept all the study procedures and protocols, and also had to sign an individual health information disclosure form to use the study data as anonymous for research. The patients who had systemic disorders or medical conditions that would affect oral health (such as HIV, diabetes) and those using drugs that cause xerostomia and un-cooperative patients were excluded from the study. Also, the patients with enamel disorders, such as fluorosis and enamel hypoplasia, were excluded from the study. In addition, the patients with severe crowding in anterior teeth were excluded because of difficulties in laser irradiation to all regions of teeth. The treatment protocols were not important in the patient selection and included extraction or non-extraction treatments.
In this study, 584 teeth from 100 patients were included. Five patients (two patients from the laser group and three from the control group) dropped from the study (four patients moved out of the city and one died). Ultimately, 554 teeth from 95 patients were included in this study. Among them, 35 were male and 60 were female. An informed consent was taken from each patient. The patients were randomly allocated to two groups:
- Laser group (278 teeth from 48 patients)
- Control groups (276 teeth from 47 patients)
Randomization and Blinding
Stratified randomization was performed by permuted blocks based on age (12–20.99 and 21–30 years) and gender. A block size 4 was placed in an envelope. The envelopes were numbered and sealed, and the principal investigator performed the randomization. The patients, the data analyst, and the observers performing the measurements during 6 months were kept blinded to the treatment.
Bonding the Brackets
At the baseline, the teeth were cleaned and polished by water slurry of pumice and rubber cup. Afterwards, the teeth were isolated and treated with 37% phosphoric acid (MorvaEtch, Iran) for 20s. Enamel surfaces were rinsed with distilled water for 15s, and dried with air spray for 15s to remove acid etching gel completely. Adhesive bonding agent (AdperTM single bond, 3M ESPE, USA) was applied on the enamel surfaces according to the manufacturer’s instruction, and then was cured for 20s using a light curing unit (Kerr, Orange, Kalif). Fluoride-free Transbond XT resin composite (3M Unitek, Monorovia, California, USA) was applied, and stainless steel brackets with slot size of 22 (3M Unitek, Monorovia, California, USA) were placed while excess composite was removed. Resin composites were cured for 20s (Kerr, Orange, Kalif) with a light intensity of 650 mW/cm2 from occlusal, gingival, mesial, and distal directions. Finally, the teeth were desiccated with air spray to identify and record any WSLs according to the scoring index.
Laser and Sham Light Irradiation
In the laser group, the maxillary anterior teeth were exposed to CO2 laser (10.6 μm wavelength, 0.4 mw power, 5 Hz frequency, 0.2 mm diameter, and 9 s pulse time). Laser irradiation was performed by one operator (SK) for 20 s with 5 mm distance from the buccal surface and constant backward and forward movements. Sham light was also irradiated to the samples of the control group with the same protocol. We used Sham light as a placebo light.
To control oral hygiene and other risk factors in both groups, the patients were advised to brush their teeth with a soft toothbrush and fluoridated toothpaste and dental floss twice a day (Crest, 1100 ppm F). Also, they were instructed to avoid the acidic food or drinks and too much sugar.
Data Collection Tool
The patients were recalled 6 months post-irradiation, and the incidence, extent, and severity of the lesions were assessed. The data were recorded in two steps:
At the base line: one week after brackets bonding
Six months later
To avoid false-positive results, the baseline assessment was performed one week after the brackets bonding. During this time, chalky appearance of enamel due to acid etching is disappeared. In each stage, the teeth were examined for the incidence, extent, and severity of WSLs, and several photographs were prepared in baseline and after 6 months. The photographic protocols were standardized and performed by one operator (SK). Five intra-oral photographs (one of the central regions, two of the right and left laterals, two of the right and left canines) were obtained by an SLR camera (Canon 550D, resolution: 18.0 megapixels) with standardized dpi, shade, color, and light. All images were taken from one angle that was perpendicular to the center of the brackets.
- WSLs’ incidence
Visual inspection was carried out by two blinded observers. The number of teeth and regions with WSLs at baseline was subtracted from those with WSLs after 6 months, and the differences demonstrated the incidence.
- WSLs’ extent
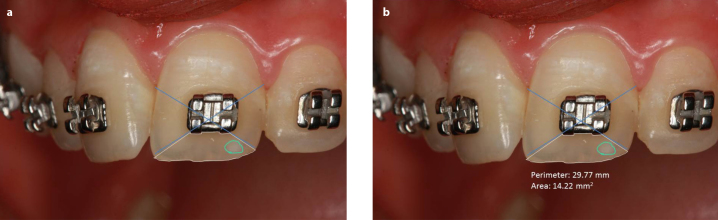
Enamel decalcification index score was used to measure the extent of the lesions (12) (Figure 1). First, the tooth surface was divided into four regions: incisal (i), mesial (m), gingival (g), and distal (d). WSLs were measured by the Digimizer software, and the proportion of each defect was calculated by dividing it into the total surface area. Two observers calculated the extent of the defects for each tooth region by enamel decalcification index score. After measuring the extent of the defects, they were scored as follows: no decalcification (0), decalcification <50% (1), decalcification >50%, (2) and 100% decalcification (3) (Figure 2, 3). The overall score of each tooth was calculated at each time-point, and then the 6-month scores were subtracted from the baseline to measure the change in the lesions.
Figure 1.
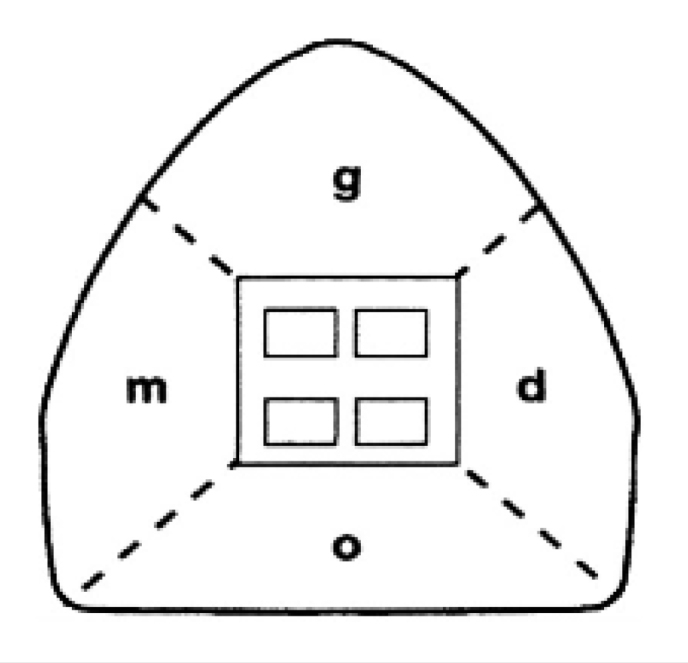
In enamel decalcification index score the tooth surface was divided into four regions occlusal (o), mesial (m), gingival (g), and distal (d); and each region was scored; no decalcification (0), decalcification <50% (1), decalcification >50%, (2) and 100% decalcification (3).
Figure 2. a, b.
Defining the tooth surface (a), defining the white spot lesion (b)
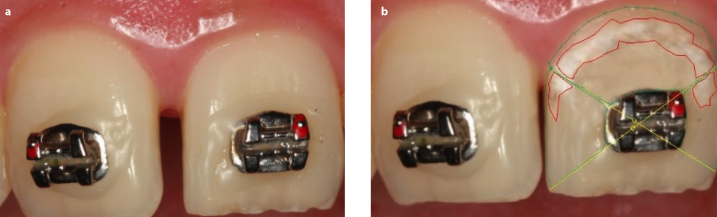
Figure 3. a, b.
Without WSL at baseline (a), with WSL at 6 months follow-up (b)
- WSLs’ severity
Clinical assessment and caries severity was measured by the following scoring index (13):
0: lack of WSL or any surface roughness (lack of demineralization)
1: WSL without any surface irregularity (mild demineralization)
2: WSL with rough surface but no restoration is required (intermediate demineralization)
3: WSL requiring restorative treatment (severe demineralization)
Each tooth was assigned an overall score, and the differences between the baseline and 6-month values were calculated.
Sample Size
To determine the sample size for each group, a priori power analysis was conducted as follows:
p1: 26% of the lesion incidence in the control group
p2: 16% of the lesion incidence in the test group
The data for this power analysis were obtained from a previous study (14). Significance level was considered as 95% and power was 80%. By inserting the minimum values in the above formula, the sample size was calculated as 548 teeth (274 per group).
Data Analysis
The development of WSLs between two time points was measured by logistic regression (generalize estimating equations, GEE). The inter-group comparison was performed by the Mann–Whitney test. The incidence, severity, and extent of the lesions in two time points in each group were compared by the McNemar analysis. The correlation between each tooth (central, lateral, or canine) and risk of WSL development in every individual overtime was assessed by GEE analysis (generalized estimating equations). All analyses were performed by Statistical Package for Social Sciences version 22.0 (IBM Corp.; Armonk, NY, USA), and significance level was considered <0.05. In addition, the inter-observer reliability and agreement was estimated by Kappa.
RESULTS
No significant difference was observed between the two study groups (p>0.05). The type of teeth had no significant effect on development of WSLs (p>0.05), whereas there were significant differences between two time points (p=0.005).
A significant difference was observed in incidence of WSLs between baseline and 6 months post-irradiation in the control group (p<0.05); however, there was no significant difference in the laser group (p>0.05) (Table 1, 2). Also, the region of tooth surface had a significant effect on WSLs (Table 3). Laser exposure was significantly effective on incisal, mesial, and distal regions (p<0.05), whereas it was not effective in the gingival sites (p>0.05) (Table 3, 4). The inter-observer agreement was estimated almost perfect (Kappa=99%).
Table 1.
Incidence of WSLs in the laser and control groups at baseline and 6 months post-irradiation. Negative values show reduction in the incidence of lesions, and positive values show an increase in the incidence of lesions. The Mann-Whitney U test was used to compare the changes in the groups
| Group | 6-month baseline | WSL+ | WSL− | Total | p |
|---|---|---|---|---|---|
| Laser | WSL+ | 29 (10.4%) | 9 (3.2%) | 38 (13.7%) | 1.00 |
| WSL− | 10 (3.6%) | 230 (82.7%) | 240 (86.3%) | ||
| Total | 39 (14%) | 239 (86%) | 278(100%) | ||
| Control | WSL+ | 18 (6.5%) | 0 (0%) | 18 (6.5%) | <0.001 |
| WSL− | 24 (8.7%) | 234 (84.8%) | 258 (93.5%) | ||
| Total | 42 (15.2%) | 234 (84.8%) | 276 (100%) |
Table 2.
Comparison of affected teeth with WSLs in baseline and after 6 months between two study groups
| Change in WSLs’ number | |||||
|---|---|---|---|---|---|
|
|
|||||
| Group | Negative | No change | Positive | Total | p |
| Laser | 9 (3.24%) | 259 (93.16%) | 10 (3.6%) | 278 (100%) | <0.001 |
| Control | 0 (0%) | 252 (91.3%) | 24 (8.7%) | 276 (100%) | |
| Total | 9 | 511 | 34 | 554 | |
Table 3.
Comparison of WSLs’ change in different regions of tooth surface between groups
| Change in regions | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Region | Group | Negative | No change | Positive | Total | p |
| Gingival | Laser | 2 | 269 | 7 | 278 | 0.245 |
| Control | 0 | 266 | 10 | 276 | ||
| Incisal | Laser | 6 | 272 | 0 | 278 | 0.003 |
| Control | 3 | 263 | 10 | 276 | ||
| Mesial | Laser | 5 | 270 | 3 | 278 | 0.005 |
| Control | 0 | 266 | 10 | 276 | ||
| Distal | Laser | 3 | 274 | 1 | 278 | 0.02 |
| Control | 0 | 271 | 5 | 276 | ||
Table 4.
Comparison of WSLs’ extent change in different regions of tooth surface between groups
| Change in WSLs’ extent | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Region | Group | −1 | No change | +1 | Total | p |
| Gingival | Laser | 5 | 267 | 6 | 278 | 0.073 |
| Control | 0 | 267 | 9 | 276 | ||
| Incisal | Laser | 9 | 269 | 0 | 278 | <0.001 |
| Control | 0 | 266 | 10 | 276 | ||
| Mesial | Laser | 5 | 270 | 3 | 278 | 0.018 |
| Control | 1 | 266 | 9 | 276 | ||
| Distal | Laser | 3 | 275 | 0 | 278 | 0.008 |
| Control | 0 | 272 | 4 | 276 | ||
Analysis of 554 teeth at baseline estimated 22 centrals, 19 laterals, and 15 canines with WSLs. In the other words, the counts of WSLs at baseline were 38 in the laser group and 18 in the control group. After 6 months of laser irradiation, 27 centrals, 31 laterals, and 23 canines were affected. The severity changes of WSLs in different regions of tooth surfaces are shown in Table 5. Accordingly, laser exposure did not have a significant effect on the severity of lesions in the gingival and distal regions (p>0.05); however, it significantly affected the incisal and mesial regions (Table 5).
Table 5.
Comparison of WSLs’ severity change in different regions of tooth surface between groups. The negative values demonstrated a decrease in the severity of WSL and vice versa; +2 and −2 values were not detected in any group.
| Change in WSLs’ severity | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Region | Group | −3 | −1 | No change | +1 | +3 | Total | P-value |
| Gingival | Laser | - | 3 | 268 | 7 | - | 278 | 0.123 |
| Control | - | 0 | 265 | 11 | - | 276 | ||
| Incisal | Laser | - | 6 | 272 | 0 | - | 278 | <0.001 |
| Control | - | 0 | 266 | 10 | - | 276 | ||
| Mesial | Laser | 1 | 5 | 269 | 1 | 2 | 278 | 0.005 |
| Control | 0 | 0 | 267 | 9 | 0 | 276 | ||
| Distal | Laser | - | 3 | 273 | 1 | 1 | 278 | 0.058 |
| Control | - | 0 | 271 | 5 | 0 | 276 | ||
DISCUSSION
WSLs may develop 6–12 months or even one month after bracket bonding (1). Because the previous studies have shown that there was no significant difference between the number of lesions in 6 and 12 months post-bonding (13, 15), we set a 6-month time frame to study the incidence and changes of WSLs. In the control group, new WSLs were developed in 8.7% cases during 6 months. Some studies have presented similar results to ours, 10.8% (16) and 7.53% (17) WSLs during the study however; some other studies demonstrated a rate of 30% which is further than ours (18). In addition, this study demonstrated a significant difference between the two study groups in terms of WSLs incidence after 6 months. The CO2 laser application demonstrated improvement of the baseline lesions and incidence of fewer lesions during 6 months. It is assumed that laser induces the chemical change in the subsurface crystals of enamel and eliminates caries by remineralization (19).
Several studies, such as this one, have examined the effect of CO2 laser with a wavelength of 10.6 μm on preventing demineralization or increasing microhardness (20, 21). Some of them did not detect any significant change in the enamel microhardness after laser irradiation. They showed that the pulsed CO2 laser irradiation alone was not able to inhibit the surface looseness of dentin and enamel due to erosion (14, 22). On the contrary, other studies showed that CO2 laser irradiation could prevent caries lesions progression up to about 80%. They explained that this effect depended on the number of pulses used, but there was no correlation between the caries resistance and morphological changes in the enamel surface (21). CO2 laser irradiation with 10.6 μm wavelength inhibits WSLs’ development hypothetically by the prevention of demineralization, increased enamel microhardness, and acid resistance (5, 8, 19, 23, 24). This effect was explained by reduced solubility either by physical melting and fusion or by re-crystallization of the enamel (25). Several studies have also shown the positive effect of CO2 laser with other wavelengths on the enamel hardness (26, 27). On the other hand, Stangler et al. (28) and Rechmann et al. (24) exhibited that the CO2 laser irradiation around the orthodontic brackets with or without topical fluoride was effective on inhibiting caries. The controversy between our results and some studies may be explained by the differences in laser parameters, sample size, measurement methods, and inclusion criteria.
The results of this study showed that laser irradiation had no significant effect on the development of gingival lesions, whereas it was effective on the incisal, mesial, and distal regions. The extent of the lesions in the incisal, mesial, and distal regions reduced significantly after CO2 laser irradiation, whereas laser had no significant effect on the gingival region. In addition, the severity of the lesions did not change in the gingival and distal areas but significantly reduced in the mesial and incisal regions. Probably because of the structural differences and the enamel thickness in the gingival region, laser was not effective in this area (29). Because the WSLs commonly affect the gingival regions (30), the laser parameters should be changed in these areas to have a positive effect on the reduction of these lesions. Also, the oral hygiene improvement could reduce the incidence of the gingival lesions (due to more plaque accumulation).
Some studies have reported that caries susceptibility increased significantly in pre-adolescent (≤16 years) when compared to adolescents (>16 years) (17, 31–33). But in this study, selecting or randomization based on the age was only performed for normal distribution of the patients in both groups. Also, the used CO2 laser parameters in this study were 10.6 μm wavelength, 0.4 mw power, 5 Hz frequency, and 9 s pulse time. In the previous study, laser etching of enamel surface by CO2 laser at 3 We showed an increased temperature of 3.5°C that was within the acceptable physiologically limitations of the pulp (33). Also, in another study, all irradiated samples with pulsed CO2 laser at 10.6 μm wavelength, and 2, 4, 6, 8, and 10 watts power showed an increased intrapulpal temperature below 3°C (34).
This study was novel in the field of clinical assessment of CO2 laser effect on the incidence, extent, and severity of WSLs in four regions of the tooth surfaces. Co-application of fluoride with CO2 laser or irradiation with various laser parameters may be interesting topics for future studies. One limitation of this study was that the demineralization scoring was assessed subjectively. Nowadays, technology offers more objective solutions to caries assessment like diagnodent. But because of the high cost, we did not have access to it.
CONCLUSION
Incidence of WSLs was significantly different between baseline and 6 months post-irradiation in the control group; however, there was no significant difference in the laser group.
WSLs changes were affected by the region of tooth surface. Laser exposure was significantly effective on incisal, mesial, and distal regions, whereas it was not effective in the gingival sites.
Laser exposure did not have a significant effect on the severity of the lesions in the gingival and distal regions; it significantly affected the incisal and mesial areas.
Acknowledgment
This research was founded and supported by a grant from Hamadan University of Medical Sciences. The results described in this paper were part of a post graduate thesis in orthodontic Dentistry.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Research Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (ID: IR.UMSHA.REC.1396.146)
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.M., S.K.; Design - M.M., L.R.S., S.K.; Supervision - M.M., L.R.S.; Materials - S.A.; Data Collection and/or Processing - S.K.; Analysis and/or Interpretation - M.F., S.K.; Literature Search - F.N.; Writing Manuscript - F.N., S.K.; Critical Review - M.M., S.A., M.F., F.N.; Other - M.F., L.R.S., S.A.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This research was founded and supported by a grant from Hamadan University of Medical Sciences. The results described in this paper were part of a post graduate thesis in orthodontic Dentistry.
REFERENCES
- 1.Gavrilovic I. White Spot Lesions in Orthodontic Patients: Formation. Prevention and Treatment. J Oral Hyg Health. 2014;2:1–3. [Google Scholar]
- 2.Øgaard B, Larsson E, Henriksson T, Birkhed D, Bishara SE. Effects of combined application of antimicrobial and fluoride varnishes in orthodontic patients. Am J Orthod Dentofacial Orthop. 2001;120:28–35. doi: 10.1067/mod.2001.114644. [DOI] [PubMed] [Google Scholar]
- 3.Rolla G, Øgaard B. Orthodontic Materials: Scientific and Clinical Aspects. Stuttgart, Thieme; 2001. Oral microbiological changes, long-term enamel alterations due to decalcification, and caries prophylactic aspects; pp. 123–42. [Google Scholar]
- 4.Øgaard B. Prevalence of white spot lesions in 19-near-olds: A study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989;96:423–7. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 5.Patel CKN. Selective Excitation Through Vibrational Energy Transfer and Optical Maser Action in N2-CO2. Phys Rev Lett. 1964;13:617. doi: 10.1103/PhysRevLett.13.617. [DOI] [Google Scholar]
- 6.Esteves-Oliveira M, Pasaporti C, Heussen N, Eduardo C, Lampert F, Apel C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J Dent. 2011;39:414–21. doi: 10.1016/j.jdent.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues LK, Nobre dos Santos M, Pereira D, Assaf AV, Pardi V. Carbon dioxide laser in dental caries prevention. J Dent. 2004;32:531–40. doi: 10.1016/j.jdent.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Paulos RS, Seino PY, Fukushima KA, Marques MM, de Almeida FCS, Ramalho KM, et al. Effect of Nd: YAG and CO2 Laser Irradiation on prevention of enamel demineralization in orthodontics: in vitro study. Photomed Laser Surg. 2017;35:282–6. doi: 10.1089/pho.2016.4235. [DOI] [PubMed] [Google Scholar]
- 9.Poosti M, Ahrari F, Moosavi H, Najjaran H. The effect of fractional CO2 laser irradiation on remineralization of enamel white spot lesions. Lasers Med Sci. 2014;29:1349–55. doi: 10.1007/s10103-013-1290-9. [DOI] [PubMed] [Google Scholar]
- 10.Ramalho KM, Eduardo Cde P, Heussen N, Rocha RG, Lampert F, Apel C, et al. Protective effect of CO2 laser (10.6 μm) and fluoride on enamel erosion in vitro. Lasers Med Sci. 2013;28:71–8. doi: 10.1007/s10103-012-1071-x. [DOI] [PubMed] [Google Scholar]
- 11.Mathew A, Reddy NV, Sugumaran D, Peter J, Shameer M, Dauravu LM. Acquired acid resistance of human enamel treated with laser (Er: YAG laser and CO2 laser) and acidulated phosphate fluoride treatment: An in vitro atomic emission spectrometry analysis. Contemp Clin Dent. 2013;4:170–5. doi: 10.4103/0976-237X.114864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson MA, Kau CH, English JD, Lee RP, Powers J, Nguyen JT. MI Paste Plus to prevent demineralization in orthodontic patients: a prospective randomized controlled trial. Am J Orthod Dentofacial Orthop. 2011;140:660–8. doi: 10.1016/j.ajodo.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Tufekci E, Dixon JS, Gunsolley J, Lindauer SJ. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81:206–10. doi: 10.2319/051710-262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner-Oliveira C, Nobre-dos-Santos M, Zero DT, Eckert G, Hara AT. Effect of a pulsed CO2 laser and fluoride on the prevention of enamel and dentine erosion. Arch Oral Biol. 2010;55:127–33. doi: 10.1016/j.archoralbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Lucchese A, Gherlone E. Prevalence of white-spot lesions before and during orthodontic treatment with fixed appliances. Eur J Orthod. 2012;35:664–8. doi: 10.1093/ejo/cjs070. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–8. doi: 10.1016/0002-9416(82)90032-X. [DOI] [PubMed] [Google Scholar]
- 17.Julien KC, Buschang PH, Campbell PM. Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthod. 2013;83:641–7. doi: 10.2319/071712-584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boersma J, Van der Veen MH, Lagerweij MD, Bokhout B, Prahl-Andersen B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res. 2005;39:41–7. doi: 10.1159/000081655. [DOI] [PubMed] [Google Scholar]
- 19.Fried D, Ragadio JN, Akrivou M, Featherstone JD, Murray MW, Dickenson KM. Dental hard tissue modification and removal using sealed transverse excited atmospheric-pressure lasers operating at lambda=9.6 and 10.6 microm. J Biomed Opt. 2001;6:231–9. doi: 10.1117/1.1344192. [DOI] [PubMed] [Google Scholar]
- 20.Lakshmi A, Shobha D, Lakshminarayanan L. Prevention of caries by pulsed CO2 laser pre-treatment of enamel: an in-vitro study. J Indian Soc Pedod Prev Dent. 2001;19:152–6. [PubMed] [Google Scholar]
- 21.Kantorowitz Z, Featherstone JD, Fried D. Caries prevention by CO2 laser treatment: dependency on the number of pulses used. J Am Dent Assoc. 1998;129:585–91. doi: 10.14219/jada.archive.1998.0276. [DOI] [PubMed] [Google Scholar]
- 22.Mirhashemi AH, Hakimi S, Ahmad Akhoundi MS, Chiniforush N. Prevention of enamel adjacent to bracket demineralization following carbon dioxide laser radiation and titanium tetra fluoride solution treatment: an in vitro study. J Lasers Med Sci. 2016;7:192–6. doi: 10.15171/jlms.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tepper SA, Zehnder M, Pajarola GF, Schmidlin PR. Increased fluoride uptake and acid resistance by CO2 laser-irradiation through topically applied fluoride on human enamel in vitro. J Dent. 2004;32:635–41. doi: 10.1016/j.jdent.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Rechmann P, Charland DA, Rechmann BM, Le CQ, Featherstone JD. In-vivo occlusal caries prevention by pulsed CO2-laser and fluoride varnish treatment-A clinical pilot study. Lasers Surg Med. 2013;45:302–10. doi: 10.1002/lsm.22141. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DG, Wefel JS, Jongebloed WL, Featherstone JD. Morphology, histology and crystallography of human dental enamel treated with pulsed low-energy infrared laser radiation. Caries Res. 1987;21:411–26. doi: 10.1159/000261047. [DOI] [PubMed] [Google Scholar]
- 26.Miresmaeili A, Farhadian N, Rezaei-Soufi L, Saharkhizan M, Veisi M. Effect of carbon dioxide laser irradiation on enamel surface microhardness around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2014;146:161–5. doi: 10.1016/j.ajodo.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues L, Dos Santos MN, Featherstone J. In situ mineral loss inhibition by CO2 laser and fluoride. J Dent Res. 2006;85:617–21. doi: 10.1177/154405910608500707. [DOI] [PubMed] [Google Scholar]
- 28.Stangler LP, Romano FL, Shirozaki MU, Galo R, Afonso AM, Borsatto MC, et al. Microhardness of enamel adjacent to orthodontic brackets after CO2 laser irradiation and fluoride application. Braz Dent J. 2013;24:508–12. doi: 10.1590/0103-6440201302292. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker D. Structural variations in the surface zone of human tooth enamel observed by scanning electron microscopy. Arch Oral Biol. 1982;27:383–92. doi: 10.1016/0003-9969(82)90147-9. [DOI] [PubMed] [Google Scholar]
- 30.Årtun J, Brobakken BO. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod. 1986;8:229–34. doi: 10.1093/ejo/8.4.229. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JA, Roberts WE, Eckert GJ, Kula KS, González-Cabezas C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2010;138:188–94. doi: 10.1016/j.ajodo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Al Maaitah EF, Adeyemi AA, Higham SM, Pender N, Harrison JE. Factors affecting demineralization during orthodontic treatment: a post-hoc analysis of RCT recruits. Am J Orthod Dentofacial Orthop. 2011;139:181–91. doi: 10.1016/j.ajodo.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Knösel M, Klang E, Helms H-J, Wiechmann D. Occurrence and severity of enamel decalcification adjacent to bracket bases and sub-bracket lesions during orthodontic treatment with two different lingual appliances. Eur J Orthod. 2015;38:485–92. doi: 10.1093/ejo/cjv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner-Oliveira C, Rodrigues LK, Soares LE, Martin AA, Zezell DM, Nobre-Dos-Santos M. Chemical, morphological and thermal effects of 10.6-μm CO2 laser on the inhibition of enamel demineralization. Dent Mater J. 2006;25:455–62. doi: 10.4012/dmj.25.455. [DOI] [PubMed] [Google Scholar]