Abstract
Introduction:
Obstructive sleep apnea (OSA) is a highly prevalent condition that is viewed as a major global health concern, while affecting approximately 10% of the middle-aged population. OSA is a chronic disease that has been conclusively associated with poor quality of life, cognitive impairments and mood alterations, enhanced cardiovascular and metabolic morbidity, thereby leading to marked increments in healthcare costs.
Areas covered:
The authors have reviewed the current evidence on the pathophysiology of OSA and its consequences, the heterogeneity of its phenotypic expression, the current therapeutic applications and their efficacy, and the implications for diagnosis, treatment and follow-up strategies in the context of the clinical management of OSA.
Expert commentary:
Personalized medicine in OSA identifies different needs and approaches: i) phenotyping and defining the different and segregated clusters of OSA patients whose recognition may improve prognostic predictions and guide therapeutic strategies; ii) to further characterize and predict the impact of OSA and its treatment, particularly revolving around mortality and the processes closely related to ageing (cardiovascular diseases, cancer and neurocognitive diseases); iii) the introduction of new technologies including telemedicine that have shown promise in the implementation of personalized medicine approaches.
Keywords: Sleep apnea, OSA, CPAP, Precision medicine, Personalized medicine, Sleep health
1.-. Introduction
Sleep is an essential biological function with important implication for organismal and cellular bioenergetics, particularly involving recovery, conservation and survival. Good quality and quantity of sleep are essential to maintain good health and quality of life. However, millions of people do not get enough sleep. Chronic sleep loss is a pervasive feature in today’s society lifestyles, with little priority being given to the adverse effects associated with habitual sleep restriction. The average sleep duration has fallen below 7 hours per night, reflecting a ~2 hour decline in sleep duration over the last century, that appears to be primarily driven by environmental (e.g., artificial light exposure) and social changes. Sleep duration is not only a habit that people can adopt of their free will, but also one that can be influenced by physical, mental, work, and many other social situations. The detrimental health effects of chronic sleep loss have now been extensively and conclusively recognized in numerous epidemiological studies [1]. However, in addition to the adverse impositions of sleep restriction, people suffering from a variety of sleep disorders are also at increased risk of poor job performance, obesity and premature mortality
Among the myriad of sleep disorders, sleep-disordered breathing (SDB) is one of the most prevalent conditions that compromise the quality and duration of sleep. The main condition in SDB-affected patients is obstructive sleep apnea (OSA), a disorder that occurs at all ages across the lifespan, and affects at least 10% of the middle-age population[2–4]. OSA imposes important pathophysiological consequences that lead to quality of life deterioration, increased motor vehicle accidents, depression and cognitive impairments, cardiovascular and metabolic disease, and, more recently, even cancer[5,6]. The clinical manifestations of OSA are markedly heterogeneous, such that two patients with the same disease severity as defined by their diagnostic polysomnographic studies will not necessarily manifest similar presenting symptoms or consequences. Although widely recognized and acknowledged, the vast heterogeneity in the clinical presentation of OSA and the divergences encountered in the responses to the traditional and validated OSA treatments have not been systematically characterized. Thus, identification of the different phenotypes of OSA constitutes a first step in the development and implementation of personalized medicine. In this context, realistically applicable methods enabling correct and accurate characterization of the different phenotypes of OSA and adequate selection of the optimal therapeutic option for each specific phenotype are currently major priorities in the field.
2.-. Obstructive sleep apnea
Obstructive sleep apnea (OSA), is a common and prevalent disorder affecting at least 10% of the middle-age population, although wide age, sex, and ethnic variations are reported globally [2–4]. Obstructive sleep apnea events reflect the collapse of the upper airway during sleep. These events lead to intermittent hypoxemia and hypercapnia, and are usually accompanied by cortical arousals potentially leading to daytime hypersomnolence and poor quality of life. The pathophysiology of OSA is multifactorial, and involves the interactions between reductions in upper airway dimensions, such as induced by obesity or maxillofacial structural changes, and the presence of increased pharyngeal collapsibility owing to reduced neuromuscular compensation and lack or attenuation of the pharyngeal protective reflexes during sleep [7].
Although OSA can be completely asymptomatic, when clinical symptoms emerge, they are characterized by intermittent snoring, unrefreshing sleep, and daytime sleepiness. OSA is an important public health issue because it is associated with development or accelerated progression of cardiovascular events, increased risk for metabolic diseases, sexual dysfunction, reduced job performance, overall negative effects on quality of life, and has been implicated as playing a causative role in traffic accidents. Among OSA patients, only a small proportion are currently diagnosed and treated. This major shortfall has direct consequences on public health overall economic impact in light of the high financial healthcare and societal costs of untreated OSA.
The events associated with collapse of the upper airway lead to brain arousal, changes in intrathoracic pressures, and intermittent episodes of hypoxemia and re-oxygenation. These events take place in repetitive cycles during sleep and induce the activation of various pathways (intermediate mechanisms) that for example predispose to atherosclerosis. Basic research and epidemiological and clinical data support the notion that OSA has a role in the initiation or progression of several cardiovascular diseases[5]. Although the mechanisms underlying the initiation and aggravation of cardiovascular disease have not been fully elucidated, several pathogenic factors are proposed as the intermediate mechanisms linking OSA with cardiovascular disease, mainly oxidative stress, sympathetic activation, inflammation, hypercoagulability, mitochondrial dysfunction, and endothelial dysregulation. Some of these pathogenic factors have been also recently postulated as linking OSA with metabolic disease[8] and cancer[9]. Obviously, the relative contributions of each of these intermediate pathways to their corresponding effects on target organs and systems will operate as primordial determinants of the ultimate clinical phenotype presenting in the clinical setting.
3.-. Clinical faces of OSA and clustering of the disease into definable phenotypes
OSA is nowadays considered as representing a complex, heterogeneous and multicomponent pathological condition. Although the current diagnosis of OSA is based on sleep study criteria, the latter may not adequately capture the whole spectrum of disease heterogeneity. Indeed, at any level of OSA severity, as predicated on sleep study measures, the clinical phenotype may be vastly different. However, OSA heterogeneity has not been formally characterized or defined, and this lack of identifiable and classifier criteria constitutes one of the main challenges for delineation of a cogent and effective approach to OSA management. Improved phenotyping and defining the different clusters of OSA patients may improve prognosis prediction and help therapeutic strategies. Efforts in such direction are mandatory in the new era of precision medicine, where treatments are targeted to the needs of each individual patient on the basis of their genetic, biomarker, phenotypic, or psychosocial characteristics, hence distinguishing a given patient from all other patients with similar clinical presentations.
There are both structural and physiological risk factors for OSA. Structural risk factors include craniofacial dimensions[10], with shortened mandible being particularly prominent [11–14]. Increased size of upper airway soft tissue structures, such as lateral walls of the pharynx and the tongue, are also well identified risk factors[15]. Both the craniofacial risk factors for OSA and soft tissue risk factors are heritable traits. Obesity is one of the strongest risk factors for OSA, such that a 10% weight gain is associated with a six fold increase in OSA risk[16]. In fact, the metabolic syndrome occurs in about half of ‘‘real-life’’ OSA patients [17] and a shared genetic background for both OSA and for adiposity measures has been identified [18]. Growing evidence has further demonstrated familial clustering of OSA[19,20]. These studies have supported the contention positing that an underlying genetic susceptibility to OSA exists. Extensive research has also suggested that obesity has a strong genetic basis [21]. However, OSA is a complex disease, and in spite of the strong clinical and epidemiological evidence supporting the importance of several genetic factors influencing the risk of OSA, the actual genetic basis of OSA is still largely unknown. A recent study reported genome-level significant findings for OSA-related physiological traits in any population. These findings identified novel associations in inflammatory, hypoxia signaling and sleep-related pathways[22]. However, nearly 40% of the total genetic variance in OSA is shared with obesity, thereby making it arduous to detect unique genome wide identifiers for OSA that are not concurrently relevant to body weight and adiposity [18]. The current interpretation of genetic studies is therefore centered around the fact that although the strongest recognized risk factor for adult OSA is obesity, the genetic basis for OSA likely involves additional non-obesity related pathways, such as those ascribable to craniofacial anatomy, ventilatory control and sleep-wake regulation.
As mentioned above, OSA severity is traditionally classified according to the polysomnographically-derived apnea-hypopnea index (AHI). Apnea is defined as the absence of airflow for ≥10 s and hypopnea is defined as i) ≥30% airflow reduction and ≥4% desaturation or arousal, or ii) ≥50% airflow reduction and ≥3% desaturation or arousal [23]. The AHI is calculated based on the average number of apnea plus hypopnea episodes per hour of sleep during in-lab or home based polysomnography, or using total recording time when respiratory polygraphy is employed as the diagnostic method. Excessive daytime sleepiness (EDS) is one of the major symptoms of the disease and traditionally one of the preponderant drivers for seeking medical assistance. EDS is associated with reduced quality of life and increased risk for traffic, work, and domestic accidents. Because of its implications, EDS has been proposed as a marker of OSA severity, especially for cardiovascular and metabolic outcomes [24–27].
Nevertheless, less than 20% of patients have symptomatic OSA[28], such that the use of the latter as a severity criterion is more likely to fail than be useful in clinical settings. However, the dichotomous EDS presentation of the disease reiterates and strengthens the likely existence of different phenotypes. Another drawback is that although EDS is a principal clinical symptom of OSA, the severity of the disease, when assessed by the AHI, does not strongly correlate with the frequently used subjective measure of EDS[29], namely the Epworth sleep scale score (ESS)[30]. ESS is a subjective and overall extensively validated method to measure daytime somnolence, but the lack of associations between ESS values and corresponding AHI is a major detractor in the clinical application and utility of this tool.
The causes of OSA are multifactorial and not just anatomically driven. Thus, adequate classification of OSA patients based on approaches that incorporate assessments of the multifactorial contributions should enable development of novel targeted therapies for OSAn. In this setting, novel pathophysiological phenotypes that incorporate differential contributions such as passive critical closing pressure of the upper airway (Pcrit) (i.e., the collapsibility of the passive upper airway), overall loop gain (a measure of instability of the overall ventilatory control system), arousal threshold to hypoxia and hypercapnia, and reflex responses of upper airway dilator muscles to negative interstitial pressure changes should permit more precise delineation of treatment approaches. Indeed, one or more non-anatomic pathophysiologic traits are present in 69% of patients with OSA. Subjects with OSA can have different mechanisms contributing to airway obstruction [31]. Eckert et al [31] proposed the PALM (passive critical closing pressure of the upper airway (Pcrit), arousal threshold, loop gain, and muscle responsiveness) scale to assist in categorizing patients with OSA according to their anatomic and non-anatomic phenotypic traits. These four physiological traits contributing to OSA may be targeted with future personalized treatment modalities that include either isolated or combined therapies targeting the anatomy (CPAP, upper airway surgery, mandibular advancement devices, positional therapy and weight loss), loop gain (O2 and CO2, pharmacological agents, antioxidants), arousal threshold (sedatives), and upper airway recruitment threshold (hypoglossal nerve stimulation, chemical upper airway stimuli). Thus, the currently available treatment approaches and their precise matching to the underlying factors promoting OSA will undoubtedly result in much more specific and effective therapeutic choices.
Furthermore, the current situation in the process of classifying OSA patients and the necessity for treatment is far from optimal: i) a unique objective index such as the AHI for the classification of the disease severity does not reliably resolve the problem of patient classification and treatment selection; ii) a subjective index (ESS) to evaluate the main symptoms of OSA is far from being useful in practice because many patients are not affected and the ESS does not correlate with the severity of the disease. On the other hand, several studies have indicated that higher AHI values are associated with higher mortality and morbidity [32,33]. Thus, using the AHI, even in light of its inherent limitations, is an approach that remains the simplest and most effective method for classifying OSA severity. However, classifying patients solely on the basis of AHI and ESS is also insufficient since it does not capture the broad spectrum of disease phenotypes, and leads to inadequate therapeutic decisions. Efforts to performed unbiased clustering or machine-learning approaches suggest that addition of incremental parameters to the classification approaches may be of value in defining more clearly subjects with more severe disease. Indeed, studies have emerged in recent years and aimed to characterize the different phenotypes of OSA. For example, unsupervised cluster analysis was used to identify subtypes of patients who are diagnosed with a particular disorder, and is predicated on the fact that individuals are grouped together based on specified variables, so that members of each cluster are as similar as possible to others within the cluster, but as different as possible compared with those in other clusters. Ye et al [34] was one of the first studies to use cluster analysis approaches in OSA. These authors analyzed a total of 822 patients with newly diagnosed moderate-to-severe OSA from the Icelandic Sleep Apnea Cohort. They described the existence of three distinct clusters based on symptom reporting and the existence of major comorbidities. The three distinct clusters were classified as the ‘‘disturbed sleep group’’ as the group with the highest probability of experiencing insomnia-related symptoms, the ‘‘minimally symptomatic group’’ as the group of patients who were much more likely to feel rested upon waking up, and the ‘‘excessive daytime sleepiness group’’ which incorporated those patients with a significantly higher ESS score. They further found that the probabilities of having co-morbid arterial hypertension and cardiovascular disease were highest in the cluster ‘‘minimally symptomatic group’’, and lowest in the cluster ‘‘excessive daytime sleepiness group’’. Another study analyzed 198 patients with OSA [35] and also identified three different communities of OSA patients. One of these clusters was represented by younger patients with very severe OSA, higher risk of nocturnal hypoxemia, morbid obesity and high prevalence of co-morbidities such as arterial hypertension. The second cluster included patients with moderate-severe OSA and low risk of nocturnal hypoxemia, and the third cluster included older patients, either overweight or mildly obese with very severe OSA. Ascending hierarchical cluster analysis on baseline symptoms, physical examination, risk factor exposure and co-morbidities was performed in data from 18,263 participants included in the French national registry of sleep apnea [36]. In this large scale study, six clusters were identified, in which patients varied considerably in age, sex, symptoms, obesity, co-morbidities and environmental risk factors. Cluster 1 included young symptomatic patients without co-morbidities; cluster 2 reflected elderly patients who were minimally symptomatic or obese OSA and few co- morbidities; cluster 3 were elderly minimally symptomatic and multi-morbid OSA; cluster 4 were young, overweight, minimally symptomatic OSA without co-morbidities; cluster 5 were middle age, with few symptoms of OSA and few co-morbidities; and cluster 6 were middle age, symptomatic multi-morbid OSA along particularly poor lifestyle habits. This study underscores the high degree of heterogeneity that exists within OSA patients regarding clinical presentation, risk factors and consequences.
A recent study [37] reported the burden of co-morbidities associated with a diagnosis of OSA, and again confirmed the different clinical presentations of OSA and the different co-morbidities associated with each OSA phenotype. This study was performed in a very large, nationwide health insurance database for working adults and retirees with employer-sponsored health insurance coverage in the USA. This database included more than 1.7 million individuals with OSA who were matched to 1.7 million controls. As with a multitude of previous studies, the authors reported that all frequent comorbidities were significantly more prevalent in OSA patients. However, sex differences were also apparent. Indeed, Type 2 diabetes and ischemic heart disease were more prevalent in men but arterial hypertension and depression were more prevalent in women with OSA. Finally, the divergence between OSA and controls was more pronounced after the sixth decade of life for most cardiovascular diseases, while depression exhibited an opposite trend.
Since one of the initial steps required to carry out a personalized medicine approach is to correctly classify the different phenotypes of a disease, there is no doubt that increased efforts at expanding the phenotypic features included in the analyses would be likely to enhance the ability to segregate the more relevant clusters and improve their predictability and usefulness. It should come as no surprise to anyone that only by properly characterizing the patient it is possible to prescribe a precise and adequate treatment.
4.-. Personalizing the diagnosis, treatment and follow-up of patients with OSA
In the current and future scenarios of financially constrained healthcare systems, in which there is already a heavy burden due to being tasked to adequately manage chronic and highly prevalent diseases such as OSA, there is also an additional estimated annual increase in the number of such cases primarily driven by aging of the population and the global increase of body mass index. As such, it becomes imperative to rationalize the delivery of care by selecting the appropriate intervention from the start for any given patient. In other words, it is necessary to develop a personalized treatment approach. To this effect, it is essential to incorporate cost effective new diagnostic, treatment and follow-up tools, together with new clinical settings, that allow an accurate and precise management of patients with chronic and prevalent diseases, reserving the allocation of very specialized and onerous care to the more complex patients who are either difficult to manage or at higher risk for morbidity and mortality.
4.1.-. Personalized diagnosis of OSA
With the aims to facilitate and expand the diagnosis of OSA to reach the affected population, it will become necessary to use simplified systems that allow for the diagnosis of a large number of patients without a strict and exclusive dependence on specialized sleep units. The gold standard for OSA diagnosis is in-laboratory polysomnography, but this method is expensive and time-consuming, which may prevent adequate attention for the large number of patients who require sleep studies.
Portable monitoring devices, designed for a less complex and faster home diagnosis, have been developed in the past two decades. The portable monitoring device approach [38], also termed respiratory polygraphy (RP), is an accepted and cheaper (20% cheaper than conventional PSG) alternative for OSA diagnosis in patients with an intermediate to high clinical probability of OSA. RP includes sensors for airflow, respiratory effort (measured with bands), and pulse oximetry readings. Masa et al[39] concluded that home RP is a comparable yet less costly alternative than polysomnography for the diagnosis and therapeutic decision making for patients with a medium to high probability of suspected OSA. This probability can be estimated through simplified methods. The Berlin questionnaire is the only OSA questionnaire developed for and validated in primary care and categorizes patients as either high or low risk for OSA based on self-reports of snoring, daytime sleepiness, hypertension and obesity[40]. The administration of a modified Berlin questionnaire prior to a sleep study could be suitable to identify high risk subjects and can thus avoid unnecessary polysomnography studies, especially in resource-limited settings[41]. Other authors have reported simplified diagnostic models consisting of a screening questionnaire followed by home oximetry that can identify patients with clinically significant OSA in a primary care population with a high degree of accuracy[42].
Finally, several studies have identified the utility of craniofacial digital photography for the diagnosis of OSA. Lee et al [43] used a model with 4 photographic measurements (face width, eye width, cervicomental angle, and mandibular length) and correctly classified 76.1% of subjects with and without OSA (area under the receiver operating characteristics curve [AUC] 0.82). Combination of photographic and other clinical data improved the prediction further (AUC 0.87). Sutherland et al[44] also found significant associations between craniofacial variable sets from facial photography and magnetic resonance imaging. Both studies concluded that facial photographic measures provide detailed anatomical data that is useful in the prediction of OSA. This approach should therefore allow for improved OSA risk stratification into craniofacial morphological phenotypes.
4.2.-. OSA treatment: Therapeutic options and differential responses to treatment
In 2006, the total direct and indirect financial cost per person of untreated OSA was estimated at €3,860 ($5,000 US/year) [45], such that treatment of OSA clearly constitutes a cost-effective intervention[46]. Management of OSA is focused on reversal of the underlying pathology. Since obesity is the main risk factor associated with the disorder, weight loss should be included as a first line intervention. Moreover, it has been recognized that sleeping in the supine position doubles a patient’s AHI compared to sleeping in the lateral or prone position, with multiple studies showing the efficacy of positional therapy. As such, patients who wear devices that alert them when adopting the supine position significantly decrease AHI[47–49].Continuous positive airway pressure (CPAP) is the most common and widely used treatment option for mild to severe OSA, and early adoption and high adherence are associated with optimal results. Since CPAP was described as a treatment of OSA in 1981[50], advances in technology have fostered the emergence of multiple approaches for delivering positive airway pressure during the night. The large number of published clinical trials investigating the efficacy of CPAP in improving different clinical OSA outcomes and the experience with millions of OSA patients who have been prescribed CPAP indicate that (i) CPAP acts as a pneumatic splint to the upper airway during sleep and corrects the obstruction in the majority of patients; (ii) adherence to CPAP is highly variable; (iii) optimal CPAP utilization and outcomes occur only in a minority of patients. As such, certain subgroups of patients, may benefit from upper airway surgical interventions or oral appliances to reverse the respiratory disturbances during sleep.
Additional options for OSA treatment include surgery, hypoglossal stimulation, and muscular training[51]. Recently, the efficacy of hypoglossal nerve stimulation has emerged. For example, Strollo et al[52] showed that hypoglossal-nerve stimulation may be an effective therapy in those with predominantly obstructive events, in whom palate collapse and supine OSA are absent. These investigators explored the effect of hypoglossal-nerve stimulation on the severity of OSA among patients who had moderate-to-severe OSA along with unfavorable response to CPAP therapy. In a highly selected group of patients with OSA, hypoglossal-nerve stimulation was associated with reductions in the frequency of respiratory events (29.3 events per hour at baseline to 9.0 events per hour at 12 months) and improvements in the oxygen desaturation index score (the number of times per hour of sleep that the blood oxyhemoglobin level dropped by ≥4% from baseline; 25.4 to 7.4 events per hour). However, only a minority of the patients with OSA is eligible for this treatment modality. Also, it is important to consider that residual disease was frequent during hypoglossal nerve stimulation therapy, as shown by the AHI, leading to the conclusion that OSA was ameliorated but not eliminated by hypoglossal-nerve stimulation. Overall, the treatment was unsuccessful in more than 30% of the patients that were implanted.
In clinical practice, intraoral mandibular advancement devices (MAD) have evolved over the years and currently constitute the second most common therapeutic choice, being the leading alternative therapy to CPAP. However, even though emergent outcomes appear to be favorable, there is less information available regarding the proportion of patients who are prescribed with MAD. As a corollary to any treatment modality for OSA, the goals are to improve symptoms and reduce the potential increase in morbidity risk associated with OSA. However, information about the effect of CPAP and MAD on the reduction of cardiovascular risk is scarce, even if many studies have assessed the effects of such interventions on OSA symptoms, with such studies having primarily focused on daytime sleepiness. Sutherland et al [53] explored the efficacy of MAD across patients with different phenotypes of OSA. This study revealed that mandibular advancement devices reduced AHI by 50.3% ± 50.7%. They also found that lower treatment response rates were more likely in supine and REM sleep. Finally they reported that in prediction modelling, age, baseline AHI, and anthropometric variables were predictive of MAD treatment outcomes, but OSA phenotype was not. Several studies have evaluated the short-term health outcomes after treatment with MAD. Philips et al [54] performed a randomized crossover controlled trial that included 126 patients with moderate-severe OSA, to compare the effects of 1 month of either CPAP or MAD treatment on cardiovascular and neurobehavioral outcomes. These authors found that CPAP was more efficacious than MAD in reducing AHI. However, they found that 24-hour mean arterial pressure response was not inferior during treatment with MAD compared to CPAP, but that overall, neither one of these two treatments yielded significant improvements in blood pressure after one month. Finally, they found that sleepiness, driving simulator performance, and disease-specific quality of life improved similarly on both treatments, although MAD was superior to CPAP in improving four general quality-of-life domains. A recent meta-analysis[55] reported that both CPAP and mandibular advancement devices are effective treatment modalities and effectively reduce daytime sleepiness in patients with OSA, with CPAP seemingly emerging as a more effective approach when compared to mandibular advancement devices.
CPAP also appeared to achieve a larger effect in more severe or sleepier OSA patients when compared with untreated controls. However, mandibular advancement devices are still viewed as a valid alternative treatment for patients with mild to moderate OSA, and as an effective alternative treatment in those case in which CPAP is not tolerated and leads to non-adherence. Poor adherence to CPAP in some patients is a drawback, and some patients will discontinue treatment due to side effects that commonly relate to mask interface problems, such as air leak, skin breakdown, claustrophobia, and mouth dryness. These side effects can be potentially addressed by changing the mask type accordingly (nasal mask vs nasal pillows vs full face- mask) and by ensuring proper mask sizing and fitting. In a subset of cases, pressure intolerance can also negatively impact patient compliance with CPAP. Bilevel PAP (BiPAP) could be useful as an adjunct to CPAP for those who have pressure intolerance. In addition to clearly identifying the need for treatment in a patient with OSA, it is necessary to choose the adequate treatment for each patient, and to prospectively prevent the emergence of the side effects that ultimately negate the potential benefits of the intervention through non utilization.
There exists a rather compelling body of evidence indicating that CPAP improves daytime sleepiness and quality of life in patients with OSA [56]. Several randomized, controlled trials have shown that adherent CPAP treatment of OSA reduces overall 24- hour blood pressure [5]. However, the positive effects of CPAP on blood pressure are not systematically observed across the broad spectrum of OSA phenotypes, and are not consistently detected even within a particular OSA phenotype. Meta-analyses of the effects of treating patients with OSA with CPAP suggest that there are moderate and variable effects of CPAP on blood pressure [57–59]. Blood pressure reductions are either modest or absent in normotensive subjects [60,61], and a randomized placebocontrolled study showed no significant changes in ambulatory blood pressure in severely hypertensive patients[62]. Thus, the a priori delineation of the optimal candidates who will benefit from CPAP therapy and achieve the desired blood pressure reductions in the context of OSA treatment is not possible at the moment, and will require much more careful and precise stratification of patient phenotypes and randomized trials within each candidate OSA phenotype.
However, there is little doubt that adherence to treatment is the key element driving a beneficial response to CPAP treatment. Several studies have demonstrated the benefits of adherent CPAP treatment in primary cardiovascular prevention among patients who also manifest excessive daytime sleepiness. However, there is still a hotly debated question as to the need to treat patients with asymptomatic OSA, and whether such treatment will result in prevention of primary cardiovascular or metabolic morbidities. Indeed, the beneficial effect of CPAP treatment by intention-to-treat on cardiovascular morbidity in asymptomatic patients has not yet been demonstrated[63,64]. In non-sleepy OSA patients, effective use of CPAP (> 4 hours per night) decreases the incidence of a composite endpoint of arterial hypertension or cardiovascular events[65]. However, the usefulness of this treatment in secondary cardiovascular prevention has not been demonstrated. The recently reported SAVE trial findings [66] in a study that constitutes the largest randomized study to date and evaluated the usefulness of CPAP in secondary cardiovascular prevention has provided several question marks that merit comment. In this study, therapy with CPAP plus usual care was compared with usual care alone, and did not prevent cardiovascular events in patients with moderate-to-severe OSA and with evidence of already established cardiovascular disease. The population of the SAVE study was comprised by 63% Asian, 25% White and 11.4% other. The participants who were assigned to CPAP adhered to the treatment for a mean of only 3.3 hours per night, which may have been insufficient to provide enough relief from OSA and affectcardiovascular outcomes. A second ongoing randomized controlled study, the ISAACC study[67], should provide a valuable contribution in the quest to determine the need to apply CPAP treatment in patients with OSA, regardless of their symptoms, for secondary cardiovascular prevention.
CPAP treatment is a chronic, long-term treatment that should be used every night, ideally for the whole duration of the sleep period, and at least > 4 hours/night to show effectiveness in the reduction of cardiovascular risk. In addition to the possible side effects associated with CPAP treatment, it is important to highlight the possible deleterious effects of inadequate compliance with CPAP therapy: Using<4 hours per night could lead not only to no changes in blood pressure but may even be associated with an increase in systemic blood pressure. As such, in a recent meta-analysis [68] Bratton and colleagues stated that CPAP was not associated with a beneficial effect on blood pressure in patients with minimally symptomatic OSA, except in those patients who used CPAP for >4 hours/night. In fact, there was some evidence suggesting an increase in systolic blood pressure in patients using CPAP <4 hours/night. Moreover, CPAP treatment also could be associated with the undesirable effect of weight gain. A recent meta-analysis showed that treatment with CPAP was associated with a slight increase in weight, further reinforcing the concept that the management of these patients should include hygienic-dietary measures to prevent the undesirable effects of weight gain in a population that is already preponderantly obese[69].
In the context of OSA and arterial hypertension, a special mention of patients with resistant hypertension appears noteworthy. On the one hand, patients with resistant hypertension are approximately 50% more likely to experience cardiovascular events than hypertensive patients who do not fulfill the established criteria for resistant hypertension, with the incidence of resistant hypertension being anticipated to increase in the coming years[70]. On the other hand, it has been reported that more than 70% of patients with resistant hypertension have OSA[71]. A recent randomized controlled trial demonstrated that among patients with OSA and resistant hypertension, CPAP treatment resulted in a significant decrease in 24-hour mean and diastolic blood pressure levels, and substantial improvements in the nocturnal blood pressure patterns [71]. Among the broad spectrum of OSA phenotypes, the group of patients with OSA and resistant hypertension emerges as one selected cluster in whom a greater benefit has been demonstrated when treatment with CPAP is implemented with the aim to decrease the elevated blood pressure levels.
However and as previously mentioned, blood pressure responses are highly variable, even when adherent use of CPAP is documented, with some patients exhibiting major reductions in blood pressure (>10 mm Hg) and others showing either unchanged or even worsening of blood pressure levels[65,72,73]. In fact, 25% to 30% of patients who use CPAP treatment for >4 h/night do not experience a positive effect on blood pressure[65,71]. The underlying causes of patient variability in response to continuous adherent use of CPAP are unknown. No clinical or anthropometrical variable has been identified to date that enables clinicians to identify those patients who will respond favorably to CPAP treatment (i.e., reduced blood pressure levels). We explored cardiovascular system-focused circulating miRNA expression in responders and non-responders to CPAP treatment on blood pressure[74]. In this study, we reported a singular cluster of circulating miRNAs that predicts blood pressure responses to CPAP treatment in patients with resistant hypertension and OSA. This specific expression profile gives a quantitative score parameter (the HIPARCO-score), which identifies those patients with resistant hypertension and OSA who will exhibit a favorable blood pressure response to CPAP. This approach represents the first validated precision medicine tool for resistant hypertension management in OSA patients. Interestingly, the study also reported that effective use of CPAP treatment in patients who respond to CPAP treatment by lowering their blood pressure is accompanied by concurrent changes in cardiovascular system-related miRNAs that may potentially influence the risk for cardiovascular disease among patients with OSA and resistant hypertension.
Due to the markedly divergent and heterogeneous response patterns to CPAP treatment in patients with OSA, and considering the low adherence to treatment that occurs in a large proportion of OSA patients, which in turn may facilitate the occurrence of undesirable deleterious effects, it becomes a priority to predict the possible response to treatment in each patient cluster, and consequently adapt the treatment modality in a personalized way. Moreover, new scenarios and algorithms of treatment modalities that combine the different available options for OSA treatment are needed[75]. In this regard, a recent study assessed the incremental effect of the combined interventions (weight loss and CPAP) when compared to each approach in isolation on cardiometabolic outcomes in patients with OSA[76]. The authors showed that the combined approach implemented for 24 weeks resulted in a larger reduction in blood pressure than either CPAP or weight loss interventions alone. Moreover, weight loss provided an incremental reduction in insulin resistance and serum triglyceride levels when combined with CPAP. Finally, adherence to a regimen of weight loss and CPAP may result in incremental reductions in blood pressure as compared with either intervention alone.
As each treatment option primarily targets only one trait causing OSA, poor efficacy for single modality therapy is likely due to the other traits not being appropriately addressed. Thus, combining therapies could theoretically improve treatment efficacy[75]. In patients exhibiting residual supine-dependent OSA with a MAD, the combination of MAD with positional therapy that avoids supine position during sleep significantly reduced AHI compared to either treatment alone [77]. Moreover, it has been described that in patients exhibiting evidence of obstruction with an oronasal mask at higher pressures than those required to resolve OSA with nasal mask, the combination of oronasal mask CPAP with MAD may effectively reduce the pressures required to resolve OSA [78]. Finally, the addition of MAD in patients exhibiting residual AHI after upper airway surgery has also been shown to reduce AHI [79]. Other potential combinatorial therapeutic approaches will have to be tested in addition to isolated interventions among the various phenotypic clusters of patient phenotypes to enable optimized outcomes and targeted personalized strategies.
It is absolutely essential and mandatory to demonstrate the exact indication for treatment in each specific patient profile with OSA. Recently, a new provocative scenario has emerged to suggest that in some specific populations, OSA could be associated with a cardioprotective role. Under such circumstances, notwithstanding the presence of closely interrelated and detrimental mechanisms that link OSA to cardiovascular disease progression, epidemiological studies are also suggesting that protective mechanisms may be activated in a particular subset of patients with OSA[80]. Berger et al. [80] demonstrated that recurrent episodes of hypoxia/re-oxygenation in patients with acute myocardial injury with mild-to-moderate sleep disordered breathing activated adaptive mechanisms that improved endothelial function, providing cardioprotection in the context of acute myocardial injury. Shah et al [81] also suggested that patients with OSA have less severe cardiac injury during an acute non-fatal myocardial infarction when compared to similar patients without OSA. The authors postulated that a cardioprotective role of sleep apnea during acute myocardial infarction via ischemic preconditioning may have become activated and superseded the detrimental inflammatory and oxidative stress that is characteristically present in patients with severe OSA.
4.3.-. Personalized follow-up of patients with OSA
A combination of a new integrated clinical program extensively involving new clinical settings for the management of OSA, together with the inclusion of simplified methods for OSA diagnosis, especially in the population with high pre-test probability of OSA, may be extensively applied to address the growing burden of OSA and long waiting lists for sleep services. In this context, the importance of good adherence to CPAP treatment is as critical as the adequate selection of the treatment of OSA. In recent years, several studies have emerged that have evaluated the usefulness of telemedicine in the management of this disease. Isetta et al[82] recently reported a randomized controlled trial comparing a telemedicine-based CPAP follow-up strategy with standard face-to-face management. The authors concluded that a telemedicine-based strategy for the follow-up of CPAP treatment in patients with OSA was as effective as standard hospital-based care in terms of CPAP compliance and symptom improvement, with comparable side effects and satisfaction rates. Moreover, this strategy had lower total costs. Telemonitoring of CPAP treatment has also been associated with reduced clinical personnel burden[83]. Other authors have assessed the usefulness of telemonitoring in the management of CPAP compliance. Turino et al[84] reported that although telemonitoring did not improve CPAP treatment adherence, it was more cost-effective than traditional face to face follow-up. Whereas telemedicine is suggested to be the cost-effective choice for OSA patients monitoring to improve CPAP adherence, its utility has not been demonstrated for other outcomes, such as the cardiovascular consequences of OSA[85].
The participatory aspects of P4 medicine [86] take the patient from a passive recipient of care to one in whch they are actively managing their own wellness. Currently, access to health applications and the web through connected mobile devices play a central role in the healthcare, particularly in the context of mobile applications (apps). The application of participatory care using a web-based technology to enhance CPAP adherence has been recently demonstrated [87]. Moreover, consumer wearables can provide patients with personalized health data, which could assist with self-diagnosis and behavioral change interventions. Many health apps are currently available for use on smartphones and tablets. This new available technology makes possible for the patient: i) to participate in their own care by screening for OSA, ii) to monitor response to therapy, and iii) to monitor their own CPAP adherence. Exisiting apps include the ability to monitor sleep, blood pressure, oxygen saturation, heart rate, and snoring. Having access to such information should enable patients to increase their participation and management of their own health care, as well as facilitate better communication with their clinicians. Moreover, the use of consumer wearables and apps for monitoring will generate unique datasets that if appropriately mined should be of great value for the clinicians and healthcare orgnaizations, and foster improvements in overall healthcare and outcomes.
4.4.-. Personalizing the management of OSA: New clinical settings
The high prevalence of OSA, its chronic indolent characteristics, and its deleterious consequences, make it unrealistic for allocation and exclusive management by specialized sleep specialists. Such as with other chronic and prevalent diseases, the comprehensive care management of OSA should be likely transferred and assigned to primary clinical settings. Previous studies have explored the possibility of incorporating such settings in the management of a frequent and chronic disease such as OSA. Recent studies have demonstrated that a new model for sleep apnea management, involving primary care settings could be a realistic and viable alternative[88,89]. In a study performed exclusively in patients with high pre-test probability of suffering from OSA, a management model implemented by primary care physicians was not only feasible, but also exhibited comparable effectiveness when compared to the management outcomes achieved by a specialist sleep unit care model [88,90]. Moreover, Antic et al[91] demonstrated that a simplified nurse-led model of care was associated with non-inferior results, including changes in the ESS scores and CPAP compliance, compared with physician-directed care in managing symptomatic moderate-severe OSA. Similarly, it was demonstrated that among patients with OSA, CPAP treatment follow-up under a primary care model favorably compared with a specialist model, and did not result in worse CPAP adherence [89].
Finally, the incorporation of primary care into the systematic management of OSA allows for improvement in capacity in the context of a chronic and frequent disease such as OSA, while reducing the burden of care in specialized units, enabling the latter to manage more complex patient profiles. This distinction highlights that the clinical setting should be personalized to the patient.
5.-. Expert comment
Precision medicine or personalized medicine is defined as the targeted prevention or treatment of individual patient needs, as determined by their genetic characteristics, biomarker profiles, phenotypic or psychosocial characteristics, all of which confer a unique phenotype to each patient. This concept implies the generation of new approaches in the diagnosis and treatment of diseases. On the one hand, it requires new diagnostic classification algorithms with increased complexity embedded layers that enable distinguishing subgroups of patients within a particular disease sub-type. On the other hand, it involves the development of new interventional approaches that are applicable to a restricted set of specific disease profiles. The latter interventions will be predictably be more specific, more effective, and less toxic, and will necessarily prevent use of any such intervention in non-responders, thereby resulting in not only improved outcomes but also in significant reductions in healthcare costs.
Personalized medicine in sleep apnea identifies different needs. First, personalizing therapeutic applications should be based on patient individualization, correctly identifying their clinical profile and needs[92]. In addition, different types of patients with sleep apnea may have different genetic and/or molecular profiles which can be used efficiently as predictive response tools to a given treatment. Furthermore, these tools based on ether biological or molecular profiling can be useful for the predictive identification of the patient. The ultimate goal of the intervention is to change the disease treatment paradigm such as to optimize patient health. As such, we must consider the possibility of applying personalized preventive measures to OSA patients with a recognized high-risk profile for the development of various co-morbidities, and the knowledge that such targeted interventions will prevent their occurrence. Finally, in this long and continuous race towards the implementation of personalized medicine, the patient’s active participation in his/her treatment is paramount. Also, the introduction of technological aspects involving telemedicine and artificial intelligence data systems may prove useful in personalized medicine (Figure 1). These developments also imply a profound transformation of clinical personnel that needs to be responsive and adaptive to technology advancements, the implementation and integration of these advances early in pre- and postgraduate training, as the acquisition of new knowledge should enable clinicians to treat diseases under a new precision medicine perspective and philosophy.
Figure 1:
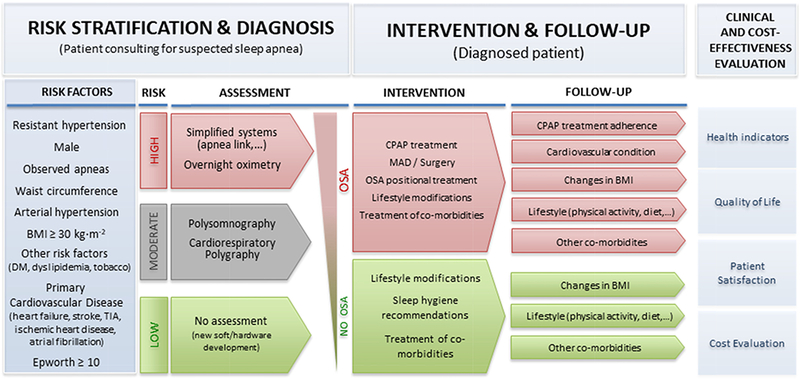
New management of OSA based on a personalized medicine approach.
There is no doubt that in order to apply precision medicine, there is a high urgency to establish a roadmap for the personalized treatment of OSA. Like any other chronic disorder, a cost-effective approach to OSA should involve all levels of care, but be principally assumed by primary care systems. This justifies the inclusion and use of simplified techniques for the diagnosis of OSA, mainly in patients with a high pre-test probability, a disruptive approach that has facilitated and expanded the diagnosis of OSA, a clearly underdiagnosed disease. In addition, we already have identified initial model tools based on the application of biomarker profiles that allow us to identify whether a particular patient will respond adequately to the treatment of OSA. It is a priority to develop additional similar instruments to predictively determine response to treatment in defined patient phenotypes.
The usefulness of CPAP has been clearly demonstrated in the treatment of symptomatic patients. However, its usefulness in minimally symptomatic or asymptomatic patients has yet to occur. Again, the profile of patients with OSA largely defines the usefulness of CPAP treatment. Thus, while CPAP has been identified as an effective treatment to control blood pressure in patients with resistant hypertension and sleep apnea[71], the usefulness of this treatment has not been demonstrated in other profiles[65] [66]. In addition, several studies have shown that CPAP could be associated with undesirable effects[68,69].Therefore, identifying the OSA patients who not only would benefit from treatment, but also those who will experience adverse effects of the treatment, constitutes another priority. Among the therapeutic alternatives, mandibular advancement devices have already begun to demonstrate their usefulness in certain profiles of OSA patients[55]. Incorporating technological and telemedicine aspects into OSA comprehensive treatment models is essential. This will enable the implementation of personalized and cost-effective treatments that will improve selection of the appropriate treatment procedure, maximize its adherence, and increase the efficacy of morbidity prevention.
6.-. Five-year view
Chronic diseases considerably increase the number of deaths worldwide. Obstructive sleep apnea (OSA) is a major global health concern affecting approximately 10% of the middle-aged population, and is associated with increased cardiovascular and metabolic disease and overall mortality risks. OSA is nowadays considered a complex, heterogeneous and multi-component disorder. Classification of OSA is based on sleep study criteria that do not adequately capture the vast and complex disease heterogeneity. Accordingly, the features of OSA heterogeneity and their determinants have not yet been characterized, and this issue constitutes one of the main challenges for OSA management. Improved phenotyping of OSA patients should improve prognosis prediction and guide therapeutic strategies. Moreover, the application of technologies for the prediction of the clinical response to CPAP treatment, such us HIPARCO-score[74], are urgently needed. Such efforts appear essential in this new era of precision medicine, whereby treatments are targeted to the needs of each individual patient on the basis of genetic, biomarker, phenotypic, or psychosocial characteristics. Effective use of resources may be best achieved by personalizing management.
OSA management is challenging for health systems; it is currently expensive and reliant on specialized personnel that is not widely available. In addition, waiting lists for sleep physician consultation and laboratory-based polysomnography are often long. Thus, there is increasing interest in the use of screening questionnaires, home sleep monitoring and ambulatory management of OSA. As a chronic disease, management of OSA should involve all healthcare levels. Treating OSA with CPAP improves symptoms and quality of life, decreases traffic accidents and may positively affect cardiovascular and metabolic morbidity. However, the effectiveness of CPAP treatment is directly related to adherence. Therefore, supervised follow-up is required for improving adherence. The number of patients undergoing CPAP treatment has increased consistently in recent years, which has overloaded follow-up resources. To guarantee effective, efficient and integral patient care and management, alternatives to such traditional methods must be entertained. These alternatives should be cost-effective and incorporate new approaches for a coordinated-care management and follow-up systems including the participation of patients, telemedicine, and smart technologies. Accordingly, innovative and cost-effective regionally adaptive models for integral, precise and personalized care management of OSA should be developed.
Moreover, OSA is also a prevalent condition in children which greatly differs from that of adults in its clinical presentation and morbidities, some of which may have long-term implications, well into adulthood[93]. As such, parallel efforts will also need to be considered in the early age groups to better understand risk stratification and operational optimization of care delivery in the pediatric age range [94–96].
Our society has already identified balanced diet, moderate physical activity and emotional well-being as the 3 pillars of healthy living. Healthy sleep should be incorporated as the fourth pillar, as clearly supported by the extensively available scientific evidence. However, the systems designed at protecting the right and the need to sleep well are not yet integrated into our lives. Once such goal is achieved, sleep and its disorders will be truly personalized.
7.-. Key issues.
-
-
Chronic sleep loss is a pervasive feature in today’s society lifestyles, with little priority being given to the adverse effects associated with habitual sleep restriction.
-
-
Sleep duration is not only a habit that people can adopt of their free will, but also one that can be influenced by physical, mental, work, and many other social situations.
-
-
The predominant condition in sleep-disordered breathing-affected patients if obstructive sleep apnea, a disorder that occurs at all ages across the lifespan, and affects at least 10% of the middle-age population.
-
-
OSA imposes important morbid consequences that lead to quality of life deterioration, increased motor vehicle accidents, depression and cognitive impairments, cardiovascular and metabolic disease, sexual dysfunction, and, more recently, even cancer.
-
-
Realistically applicable methods enabling correct and accurate characterization of the different phenotypes of OSA and adequate selection of the optimal therapeutic options for each specific phenotype are currently major priorities in the field.
-
-
OSA phenotypic heterogeneity has not been formally characterized, and the current lack of identifiable and classifier criteria constitutes one of the main challenges for delineation of a cogent and effective approach to OSA management. Improved phenotyping and defining the different clusters of OSA patients may improve prognostic prediction and help in formulation of therapeutic strategies.
-
-
Classifying OSA patients solely on the basis of apnea-hypopnea index and Epworth sleep scale scores is insufficient, since it does not capture the broad spectrum of disease phenotypes, and leads to inadequate therapeutic decisions.
-
-
Like any other chronic disorder, a cost-effective approach to OSA should involve all levels of care, but be principally assumed by primary care systems.
-
-
The incorporation of technological and telemedicine aspects into OSA comprehensive treatment models should enable implementation of personalized and cost-effective treatments that will improve selection of the appropriate treatment procedure, maximize its adherence, and increase the efficacy of morbidity prevention.
-
-
To guarantee effective, efficient and integral patient care and management of OSA, alternatives to such traditional methods must be entertained. These alternatives should be cost-effective and incorporate new approaches for a coordinated-care management and follow-up systems including the participation of patients, telemedicine, and smart technologies.
-
-
Our society has already identified balanced diet, moderate physical activity and emotional well-being as the 3 pillars of healthy living. Healthy sleep should be incorporated as the fourth pillar, as clearly supported by the extensively available scientific evidence.
Acknowledgments
Funding
This paper was funded by National Institutes of Health grant HL-130984 (Leila Kheirandish-Gozal, Principal Investigator).
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Givens ML, Malecki KC, Peppard PE, et al. Shiftwork, Sleep Habits, and Metabolic Disparities: Results from the Survey of the Health of Wisconsin. Sleep Health. 1(2), 115–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 163(3 Pt 1), 685–689 (2001). [DOI] [PubMed] [Google Scholar]
- 3.**.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol 177(9), 1006–1014 (2013).A study of the estimated prevalence of sleep-disordered breathing in the United States for the periods of 1988–1994 and 2007–2010 that included 1,520 participants who were 30–70 years of age.
- 4.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. The Lancet Respiratory Medicine. 3(4), 310–318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. The Lancet Respiratory Medicine. 1(1), 61–72 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 187(1), 99–105 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 383(9918), 736–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.gileles-hillel A, Kheirandish-Gozal L, Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 12(5), 290–298 (2016). [DOI] [PubMed] [Google Scholar]
- 9.**.Gozal D, Farré R, Nieto FJ. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med Rev. 27, 43–55 (2016).This review describes how the connection between chronic sleep fragmentation and intermittent hypoxia, the hallmark features of OSA, is biologically plausible, describing several putative mechanisms.
- 10.Chi L, Comyn F-L, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. European Respiratory Journal. 38(2), 348–358 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilleminault C, Riley R, Powell N. Obstructive sleep apnea and abnormal cephalometric measurements. Implications for treatment. Chest 86(5), 793–794 (1984). [DOI] [PubMed] [Google Scholar]
- 12.Lowe AA, Santamaria JD, Fleetham JA, Price C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 90(6), 484–491 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome. I. Skeletal morphology. J Laryngol Otol. 103(3), 287–292 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE. Craniofacial structure and obstructive sleep apnea syndrome--a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 109(2), 163–172 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of Upper Airway Anatomic Risk Factors for Obstructive Sleep Apnea with Volumetric Magnetic Resonance Imaging. Am J Respir Crit Care Med. 168(5), 522–530 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. Jama. 284(23), 3015–3021 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Bonsignore MR, Esquinas C, Barceló A, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Respir J. 39(5), 1136–1143 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Patel SR, Larkin EK, Redline S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int J Obes (Lond). 32(5), 795–800 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 151(3 Pt 1), 682–687 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med. 122(3), 174–178 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. Jama. 256(1), 51–54 (1986). [PubMed] [Google Scholar]
- 22.Cade BE, Chen H, Stilp AM, et al. Genetic Associations with Obstructive Sleep Apnea Traits in Hispanic/Latino Americans. Am J Respir Crit Care Med. rccm.201512–2431OC-124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 32(2), 150–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapur VK, Resnick HE, Gottlieb DJ, Sleep Heart Health Study Group. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 31(8), 1127–1132 (2008). [PMC free article] [PubMed] [Google Scholar]
- 25.Lombardi C, Parati G, Cortelli P, et al. Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res. 17(3), 263–270 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax. 64(10), 834–839 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Barceló A, Barbe F, La Peña de M, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 63(11), 946–950 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 328(17), 1230–1235 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Weaver EM, Woodson BT, Steward DL. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg. 132(2), 255–262 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 14(6), 540–545 (1991). [DOI] [PubMed] [Google Scholar]
- 31.**.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 188(8), 996–1004 (2013).This sutdy contributes to the identification of OSA is a heterogeneous disorder definging the proportion of key anatomic and nonanatomic contributions in a relatively large cohort of patients with OSA and control subjects to identify pathophysiologic targets for future novel therapies for OSA.
- 32.Lavie P, Lavie L. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des. 14(32), 3466–3473 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 31(8), 1071–1078 (2008). [PMC free article] [PubMed] [Google Scholar]
- 34.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 44(6), 1600–1607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacedonia D, Carpagnano GE, Sabato R, et al. Characterization of obstructive sleep apnea-hypopnea syndrome (OSA) population by means of cluster analysis. J Sleep Res. 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Bailly S, Destors M, Grillet Y, et al. Obstructive Sleep Apnea: A Cluster Analysis at Time of Diagnosis. PLoS ONE. 11(6), e0157318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. European Respiratory Journal. 47(4), 1162–1169 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Alonso Alvarez M de LL, Terân-Santos J, Cordero Guevara J, et al. [Reliability of home respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome: analysis of costs]. Arch Bronconeumol. 44(1), 22–28 (2008). [PubMed] [Google Scholar]
- 39.Masa JF, Corral J, Sanchez de Cos J, et al. Effectiveness of three sleep apnea management alternatives. Sleep. 36(12), 1799–1807 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 131(7), 485–491 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J. Med. Res 124(3), 281–290 (2006). [PubMed] [Google Scholar]
- 42.Chai-Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care Thorax. 66(3), 213–219 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Lee RWW, Petocz P, Prvan T, Chan ASL, Grunstein RR, Cistulli PA. Prediction of obstructive sleep apnea with craniofacial photographic analysis. Sleep. 32(1), 46–52 (2009). [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland K, Schwab RJ, Maislin G, et al. Facial phenotyping by quantitative photography reflects craniofacial morphology measured on magnetic resonance imaging in Icelandic sleep apnea patients. Sleep. 37(5), 959–968 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jennum P, Kjellberg J. Health, social and economical consequences of sleep-disordered breathing: a controlled national study. Thorax. 66(7), 560–566 (2011). [DOI] [PubMed] [Google Scholar]
- 46.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 13(4), iii-iv- xi-xiv- 1–119- 143–274 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Eijsvogel MM, Ubbink R,Dekker J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. JCSM. 11(2), 139–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson M, Collins A, Berlowitz D, Howard M, O’Donoghue F, Barnes M. Efficacy of sleep position modification to treat positional obstructive sleep apnea. Sleep Med. 16(4), 545–552 (2015). [DOI] [PubMed] [Google Scholar]
- 49.van Maanen JP, Meester KAW, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 17(2), 771–779 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Sullivan C, Issa F, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1(8225), 862–865 (1981). [DOI] [PubMed] [Google Scholar]
- 51.Guimarães KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 179(10), 962–966 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 370(2), 139–149 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. JCSM. 11(8), 861–868 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 187(8), 879–887 (2013). [DOI] [PubMed] [Google Scholar]
- 55.**.Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. The Lancet Respiratory Medicine. 3(11), 869–878 (2015).In this metaanalysis the autor described that CPAP treatment seems to be the more effective treatment that MAD, being MAD treatment an alternative treatment for OSA, especially for patients with mild to moderate obstructive sleep apnoea.
- 56.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 164(4), 608–613 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Haentjens P, van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. 167(8), 757–764 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 50(2), 417–423 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 185(2), 67–72 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 165(6), 773–780 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 163(2), 344–348 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 129(6), 1459–1467 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 134(11), 1015–1023 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Robinson G, Smith D, Langford B, Davies R, Stradling J. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 27(6), 1229–1235 (2006). [DOI] [PubMed] [Google Scholar]
- 65.**.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA: The Journal of the American Medical Association. 307(20), 2161–2168 (2012).In this randomized controlled trial the authors report that in patients with OSA without daytime sleepiness, the prescription of CPAP compared with usual care did not result in a statistically significant reduction in the incidence of hypertension or cardiovascular events.
- 66.**.McEvoy RD, Antic NA, HEELEY E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 375(10), 919–931 (2016).This randomized controlled trial is the largest study to evaluate the effect of CPAP treatment in secodary cardiovascular prevention.
- 67.Esquinas C, Sánchez-de-la-Torre M, Aldomá A, et al. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol. 36(9), 495–501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bratton DJ, Stradling JR, Barbé F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. thoraxjnl-2013–204993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 70(3), 258–264 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 125(13), 1635–1642 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martínez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA: The Journal of the American Medical Association. 310(22), 2407–2415 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. Journal of Hypertension. 19(12), 2271–2277 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Muxfeldt ES, Margallo V, Costa LMS, et al. Effects of Continuous Positive Airway Pressure Treatment on Clinic and Ambulatory Blood Pressures in Patients With Obstructive Sleep Apnea and Resistant Hypertension: A Randomized Controlled Trial. Hypertension. 65(4), HYPERTENSIONAHA.114.04852–742 (2015). [DOI] [PubMed] [Google Scholar]
- 74.**.Sánchez-de-la-Torre M, Khalyfa A, Sánchez-de-la-Torre A, et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J Am Coll Cardiol. 66(9), 1023–1032 (2015).In this study the authors described the first personalized medicine tool to predict blood pressure response to CPAP treatment in patients with resistant hypertension and OSA.
- 75.Deacon NL, Jen R, Li Y, Malhotra A. Treatment of Obstructive Sleep Apnea. Prospects for Personalized Combined Modality Therapy. Annals ATS. 13(1), 101–108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, Weight Loss, or Both for Obstructive Sleep Apnea. N. Engl. J. Med 370(24), 2265–2275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 19(2), 637–644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaminska M, Montpetit A, Mathieu A, Jobin V, Morisson F, Mayer P. Higher effective oronasal versus nasal continuous positive airway pressure in obstructive sleep apnea: effect of mandibular stabilization. Can. Respir. J 21(4), 234–238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu C, Xie Y, Kang H, et al. [Efficacies of using modified oral appliance after uvulopalatopharyngoplasty in the treatment of moderate to severe obstructive sleep apnea hypopnea syndrome]. Zhonghua Yi Xue Za Zhi. 95(10), 761–765 (2015). [PubMed] [Google Scholar]
- 80.Berger S, Aronson D, Lavie P, Lavie L. Endothelial Progenitor Cells in Acute Myocardial Infarction and Sleep-disordered Breathing. Am J Respir Crit Care Med. 187(1), 90–98 (2012) [DOI] [PubMed] [Google Scholar]
- 81.Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep & breathing 17(2), 819–826 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Isetta V, Negrín MA, Monasterio C, et al. A Bayesian cost-effectiveness analysis of a telemedicine-based strategy for the management of sleep apnoea: a multicentre randomised controlled trial. Thorax. 70(11), 1054–1061 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Anttalainen U, Melkko S, Hakko S, Laitinen T, Saaresranta T. Telemonitoring of CPAP therapy may save nursing time. Sleep Breath. 20(4), 1209–1215 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Turino C, de Batlle J, Woehrle H, et al. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. European Respiratory Journal. 49(2), 1601128 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Mendelson M, Vivodtzev I, Tamisier R, et al. CPAP treatment supported by telemedicine does not improve blood pressure in high cardiovascular risk OSA patients: a randomized, controlled trial. Sleep. 37(11), 1863–1870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim DC, Sutherland K, Cistulli PA, Pack AI. P4 medicine approach to obstructive sleep apnoea. Respirology. 22(5), 849–860 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuna ST, Shuttleworth D, Chi L, et al. Web-Based Access to Positive Airway Pressure Usage with or without an Initial Financial Incentive Improves Treatment Use in Patients with Obstructive Sleep Apnea. Sleep. 38(8), 1229–1236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.**.Chai-Coetzer CL, Antic NA, Rowland LS, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA: The Journal of the American Medical Association. 309(10), 997–1004 (2013).In this randomized controlled trial the authors demosntrated that OSA management in the primary care setting is a cost-effective alternative to the specialized sleep unit setting.
- 89.Sánchez-de-la-Torre M, Nadal N, Cortijo A, et al. Role of primary care in the follow-up of patients with obstructive sleep apnoea undergoing CPAP treatment: a randomised controlled trial. Thorax. 70(4), 346–352 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 146(3), 157–166 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurseled care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 179(6), 501–508 (2009). [DOI] [PubMed] [Google Scholar]
- 92.**.Pack AI. Application of P4 Medicine to Obstructive Sleep Apnea: A Roadmap for Improving Care? Annals ATS. AnnalsATS.201604–235PS (2016).In this brilliant and inspired revision the authors identified the present and future vision of P4 medicine in OSA.
- 93.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 5(2), 274–282 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kheirandish-Gozal L, Philby MF, Qiao Z, Khalyfa A, Gozal D. Endothelial Dysfunction in Children With Obstructive Sleep Apnea Is Associated With Elevated Lipoprotein-Associated Phospholipase A2 Plasma Activity Levels. J Am Heart Assoc. 6(2), e004923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am J Respir Crit Care Med. 194(9), 1116–1126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kheirandish-Gozal L, Gozal D. Pediatric OSA Syndrome Morbidity Biomarkers: The Hunt Is Finally On! Chest. 151(2), 500–506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


