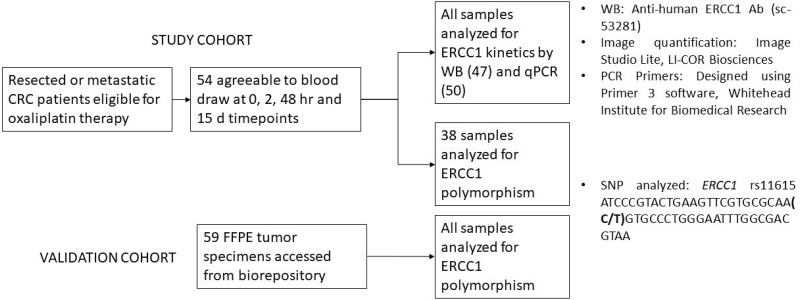
Figure 1. A schematic representation of the study workflow.
54 patients were consented for blood draw to assess ERCC1 kinetics. Expression levels in all patients were assessed by either Western blot or polymerase chain reaction. 43/54 patients had analysis by both modalities. 38/54 samples were additionally assessed for presence of ERCC1 rs11615 SNP. This was based on availability of residual sample. For validation of ERCC1 rs11615 SNP analysis 59 individual samples were additionally obtained from an institutional biorepository which stores excised tumors as Formalin Fixed Paraffin Embedded (FFPE) tissue blocks.