Figure 1.
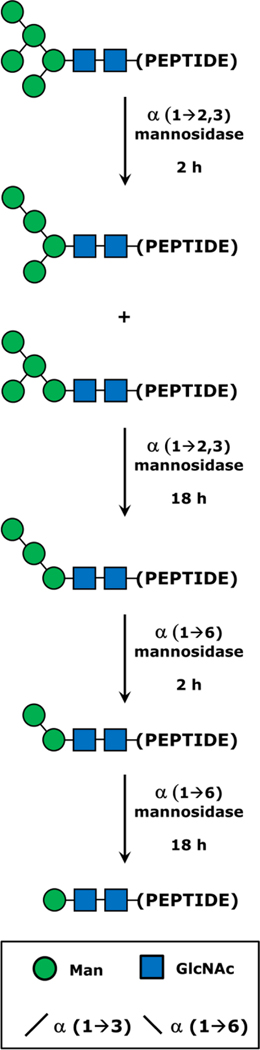
Schematic summary of the enzymatic deconstruction of N-glycan moieties to yield truncated N-glycopeptides. For simplicity, this process is illustrated starting with the smallest and most abundant RNase B glycoform, Man5. The larger N-glycans present in the mixture would be decomposed in a similar fashion. Glycopeptide preparations were first treated with α(1→2,3) mannosidase, which after a 2 h yielded a mixture of Man4 structures via hydrolysis of one of the two susceptible glycosidic linkages of Man5. With 18 h of incubation, both α(1→3) linked residues were cleaved from Man5, producing the Man3 structure. The digest was next treated with α(1→6) mannosidase, which sequentially degraded the remaining branch of Man3 to yield Man2 and Man1 after 2 h and 18 h of incubation, respectively. A key to the monosaccharide and glycosidic bond symbology is provided in the inset.
