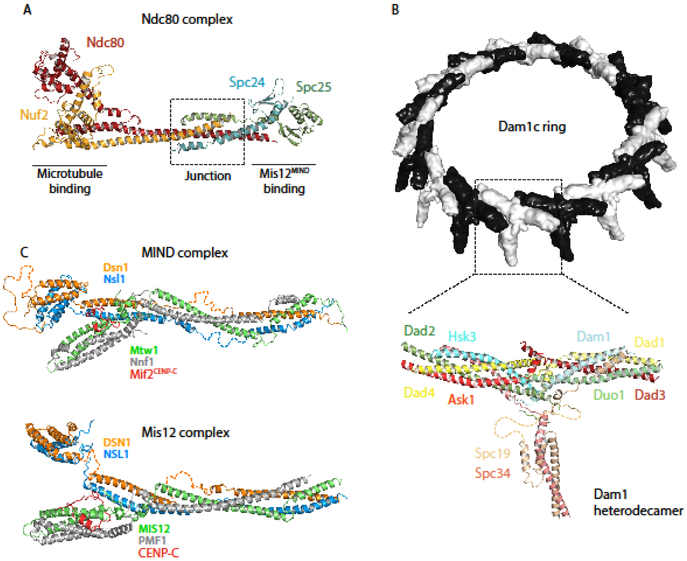
Figure 2.
Recently published structures of outer kinetochore protein complexes. (A) Ndc80cDwarf is an elongated heterotetramer comprised of a microtubule-binding Hec1Ndc80-Nuf2 dimer and Mis12MIND-binding Spc24-Spc25 dimer. The structure shows the complete junction region of the heterotetramer (PDB ID: 5TCS) [7,8]. (B) (top) Chaetomium thermophilum Dam1c heterodecamers oligomerize into 17-membered rings with an outer diameter of about 560 Å [9]. (bottom) Ribbon diagram of a heterodecamer subunit, containing amino acids 13-78 of Ask1, 18-76 of Dad1, 25-95 and 109-116 of Dad2, 18-82 of Dad3, 3-70 of Dad4, 53-107 of Dam1, 49-121 of Duo1, 22-77 of Hsk3, 7-112 of Spc19, 3-48 and 112-199 of Spc34 (PDB ID: 6CFZ) [9]. (C) (top) K.lactis MIND is a Y-shaped heterotetramer, the stem of which binds Spc24-Spc25 of Ndc80c. One globular head binds inner kinetochore proteins CENP-CMif2 and CENP-UAme1, while a N-terminal extension of Dsn1 from the other head inhibits these interactions except when phosphorylated by Aurora B kinase (PDB ID: 5T58 and 5T51) [19]. (bottom) H.sapiens Mis12c with CENP-C shares a conserved structure with its budding yeast homolog; it also forms Y-shaped heterotetramer about 200 Å long, with conserved sites of interaction with CENP-CMif2 and Ndc80(PDB ID:5LSK) [20].