Figure 1.
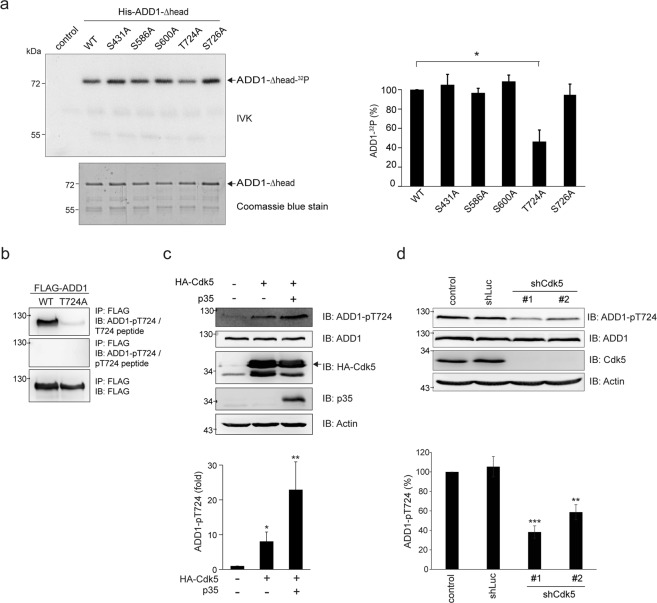
Cdk5 directly phosphorylates ADD1 at T724. (a) His-ADD1-Δhead proteins were purified and served as substrates for Cdk5/p35 complex in the in vitro kinase assay (IVK). The 32P-incorporated proteins were fractionated by SDS-PAGE and visualized by autoradiography. An equal loading of the substrate His-ADD1-Δhead was confirmed by Coomassie blue staining (lower). The phosphorylation of His-ADD1-Δhead was measured and expressed as percentage relative to the WT. Values (means ± s.d.) are from three independent experiments. *P < 0.05. (b) FLAG-ADD1 WT or T724A was transiently expressed in HEK293 cells. FLAG-ADD1 was immunoprecipitated with anti-FLAG antibody and the immunocomplexes were analyzed by immunoblotting with anti-FLAG and anti-ADD1 pT724 in the presence of 7 μM T724 phosphopeptide (pT724 peptide) or control T724 peptide. Note that the pT724 peptide successfully blocks the signal using anti-ADD1 pT724. (c) HA-Cdk5 was transiently co-expressed with (+) or without (−) its activator p35 in HEK293 cells. An equal amount of the whole cell lysates was analyzed by immunoblotting with antibodies as indicated. The level of ADD1-pT724 was measured and expressed as fold relative to the control in the absence of Cdk5 and p35. Values (means ± s.d.) are from three independent experiments. *P < 0.05; **P < 0.01. (d) MDA-MB-231 cells were infected with lentiviruses expressing shRNAs to Cdk5 (shCdk5 #1 and #2) or luciferase (shLuc) as a control. An equal amount of the whole cell lysates was analyzed by immunoblotting with antibodies as indicated. The level of ADD1-pT724 was measured and expressed as percentage relative to the control. Values (means ± s.d.) are from three independent experiments. **P < 0.01; ***P < 0.001.