Abstract
We investigated the influence of spontaneous gut leakage upon polymicrobial sepsis in a lupus model with Fc gamma receptor IIb-deficient (FcGRIIb−/−) mice aged 8 and 40 weeks, as representing asymptomatic and symptomatic lupus, respectively. Spontaneous gut leakage, determined by (i) the presence of FITC-dextran, (ii) elevated serum endotoxin, and (iii) elevated serum (1→3)-β-D-glucan (BG), was demonstrated in symptomatic lupus but not in the asymptomatic group. In parallel, spontaneous gut leakage, detected by elevated serum BG without fungal infection, was demonstrated in patients with active lupus nephritis. Gut leakage induced by dextran sulfate solution (DSS) or endotoxin administration together with BG or endotoxin alone, but not BG alone, enhanced the severity of cecal ligation and puncture (CLP) sepsis more prominently in 8-week-old FcGRIIb−/− mice. Additionally, the bone marrow-derived macrophages of FcGRIIb−/− mice produced higher cytokine levels when coexposed to endotoxin and BG, when compared to wild-type mice. In summary, spontaneous gut leakage was demonstrated in symptomatic FcGRIIb−/− mice and the induction of gut permeability worsened sepsis severity. Gut translocation of endotoxin and BG had a minor effect on wild-type mice, but the synergistic effect of BG and endotoxin was prominent in FcGRIIb−/− mice. The data suggest that therapeutic strategies addressing gut leakage may be of interest in sepsis conditions in patients with lupus.
Keywords: FcGRIIb-deficient mice, Systemic lupus erythematosus, Endogenous endotoxin, (1→3)-β-D-glucan, Sepsis severity
Introduction
Systemic lupus erythematosus (SLE) is a common autoimmune disease and has a multifactorial pathogenesis [1]. Although the influence of gut dysbiosis in the enhancement of disease progression has been demonstrated [2], data about the effects of gastrointestinal (GI) leakage in lupus are limited. Interestingly, elevated blood endotoxin, a component of the gram-negative bacterial cell wall, is common in patients with SLE [3]. As the GI tract is the endogenous source of endotoxin, endotoxemia in active lupus may be due to gut leakage. However, gut fungi are also the commensal organisms in the human GI tract [4] and (1→3)-β-D-glucan (BG) is a major component of fungal cell walls. Hence, the gut translocation of BG, a component of the contents of the intestines, may also play a role in lupus. As such, the synergistic effect of BG and lipopolysaccharide (LPS) on immune activation has been demonstrated [5]. In addition, patients with SLE are susceptible to infection [6, 7] and elevated serum endotoxin is common in such patients.
The association between Fc gamma receptor IIb (FcGRIIb) dysfunction polymorphism with SLE, particularly in Asian populations, is well known [8]. Functionally defective FcGRIIb, the only inhibitory receptor in the FcGR family, is associated with exaggerated immune responses and SLE [9]. There is a high prevalence of FcGRIIb dysfunction polymorphisms in Asian populations, possibly due to malaria-based selection pressure [10]. In host defense, the inhibitory signaling defect in FcGRIIb-deficient (FcGRIIb−/−) mice leads to the highly effective eradication of several organisms [10] and a vigorous response to endotoxin [11].
Our main purpose was to examine whether there is gut leakage in lupus and to test the possible influence of molecules derived from gut translocation, e.g., LPS and/or BG, in the severity of sepsis. Because of the high prevalence of FcGRIIb dysfunction polymorphism in Asia, we used FcGRIIb−/− mice as a representative lupus mouse model. Interestingly, FcGRIIb−/− mice on the C57BL/6 background is an established lupus mouse model [12, 13]. Lupus nephritis (LN), as demonstrated by increased anti-dsDNA (an auto antibody commonly found in lupus), elevated proteinuria, and lupus renal histopathology with immune complex deposition, could be detected as early as at the age of 24 weeks in FcGRIIb−/− mice. Most of these mice developed full-blown LN after 32–40 weeks. As such, FcGRIIb−/− mice could be used for the exploration of sepsis superimposed on lupus with either an asymptomatic or symptomatic status. In addition, for a proof-of-concept study, sepsis was induced by a cecal ligation and puncture (CLP) model. Accordingly, we explored the spontaneous elevation of serum endotoxin and BG as indicators of gut leakage in patients with active and inactive LN. We also determined the role of the synergy of serum endotoxemia and β-glucanemia in lupus, using a polymicrobial sepsis model in FcGRIIb−/− mice.
Materials and Methods
Patient Samples
To explore endotoxemia and serum BG elevation in lupus, blood and spot urine were collected from patients with LN at the King Chulalongkorn Memorial Hospital, Bangkok, Thailand. The study protocol and sample accession process were approved by the Ethical Institutional Review Board, Faculty of Medicine, Chulalongkorn University, according to the Declaration of Helsinki, with written informed consent obtained from each individual patient. All patients had documented biopsy-proven class III or IV LN according to the 2003 International Society of Nephrology/Renal Pathology Society Classification [14].
The inclusion criteria for active LN were: (1) a urine protein creatinine index of >1 g/day and (2) active urine sediments (red blood cells or white blood cells >5 cells/high-power field [HPF]). The inclusion criteria for inactive LN were: (1) a urine protein creatinine index of <0.5 g/day and (2) inactive urine sediments (red blood cells and white blood cells <5 cells/HPF). The exclusion criteria were other causes that interfered with gut leakage determination by endotoxin and BG including (1) serum creatinine >1.5 mg/dL, (2) current infections or a history of infections within 2 weeks, (3) a history of invasive fungal infection, (4) pregnancy, (5) liver injury, (6) diarrhea or a history of diarrhea within 2 weeks. The SLE Disease Activity Index 2000 (SLEDAI-2K) scoring system was used to calculate disease activity at the time of sample collection [15]. To assess proteinuria, the urine protein creatinine index (UPCI) was calculated using the following equation: UPCI = spot urine protein (mg/dL)/urine creatinine (mg/dL). Samples from healthy volunteers were analyzed for controls. The demographic data are presented in Table 1.
Table 1.
Demographic data of healthy volunteers and patients with lupus
| Patient characteristics | Healthy volunteers (n = 10) | Inactive lupus (n = 14) | Active lupus (n = 14) |
|---|---|---|---|
| Female gender, % | 100 | 100 | 100 |
| Age, years | 31±3 | 34±2 | 31±2 |
| Serum creatinine, mg/dL | 0.94±0.06 | 1.01±0.03 | 1.06±0.05 |
| Urine protein creatinine index | 0.11±0.02 | 0.52±0.12 | 1.11±0.27# |
| Urine white blood cells/mm3 | 0 | 2.77±0.53 | 41.46±15.71# |
| Urine red blood cells/mm3 | 0 | 10.23±2.09 | 30.69±6.74# |
| Patients with positive anti-dsDNA | 0 | 7 (50) | 11 (79) |
| Patients with low CH50 values | 0 | 0 | 6 (48) |
| SLEDAI-2K | 0 | 5.85±0.59 | 30.69±6.74# |
| Steroid dosage, mg/day | 0 | 5.85±0.59 | 14.15±1.91# |
Data are expressed as mean ± SE or n (%), unless otherwise indicated. SLEDAI-2K, SLE Disease Activity Index 2000.
p < 0.05 inactive versus active lupus.
The Animal Model
FcGRIIb−/− mice (C57BL/6 background) were kindly provided by Dr. Silvia Bolland (NIAID, NIH, MD, USA). Wild-type mice were purchased from the National Laboratory Animal Center, Nakornpathom, Thailand. Female mice were used in all experiments, using protocols for animals approved by the Faculty of Medicine, Chulalongkorn University, according to NIH criteria. Blood collection was performed under isoflurane anesthesia through tail vein or cardiac puncture, in the time-course observations or nonsurvival experiments, respectively. For the evaluation of immune complex deposition, GI tissue was fixed in Cryogel (Leica Biosystems, Richmond, IL, USA) and frozen with liquid nitrogen. Sections (4-µm-thick) were stained with goat anti-mouse IgG Alexa Fluor 488 (Abcam, Cambridge, MA, USA) for the detection of the Fc portion of immunoglobulin in deposited immune complexes. The fluorescence signal was detected by Zeiss LSM 800 with Airyscan (Carl Zeiss, Germany) with the excitation/emission peak at 495/519 nm.
Cecal Ligation and Puncture Surgery
A protocol of a previously described CLP procedure with a 25-gauge needle [16, 17] was performed under isoflurane anesthesia. Tramadol, 20 mg/kg diluted in 0.5 mL normal saline (NSS), was administered subcutaneously after the operation. The mice were sacrificed 18 h after CLP under isoflurane anesthesia and the serum collected was kept at −80°C until analyzed.
Dextran Sulfate Solution prior to CLP
To examine the influence of GI barrier defect (gut leakage) upon bacterial sepsis, dextran sulfate solution (DSS) was administered prior to CLP. A short course and low dose of DSS lead to asymptomatic mice with only subtle histopathology changes as previously described (data not shown) [18]. Dextran sulfate (Sigma-Aldrich, St. Louis, MO, USA) was diluted into drinking water at concentrations of 1.5% (w/v) for 1 week before the CLP procedure.
CLP with LPS and/or BG Administration
To explore the effect of endotoxin and/or BG in polymicrobial sepsis, intra-peritoneal (i.p.) LPS and/or intravenous (i.v.) BG (into the tail vein) were administered after CLP surgery. LPS of Escherichia coli 026:B6 was purchased from Sigma-Aldrich. Pachyman (Associates of Cape Cod, Falmouth, MA, USA), was used for BG [19]. Subsequently, i.p. LPS (1 mg/kg) with i.v. NSS (LPS alone), i.v. BG (50 mg/kg) with i.p. NSS (BG alone), or i.p. LPS with i.v. BG (LPS and BG in combination) was administered at 3 and 6 h post-CLP surgery.
Gut Permeability Test
Gut permeability was measured by (i) the detection, in serum, of fluorescein isothiocyanate-dextran (FITC-dextran), a nonabsorbable, high-molecular-weight molecule, after oral administration, and (ii) a spontaneous increase in serum endotoxin and BG, as previously described [18]. Briefly, for the FITC-dextran test, mice were gavaged with 0.5 mL of FITC-dextran (molecular weight, 4.4 kDa; FD4; Sigma-Aldrich) at a concentration of 25 mg/mL in sterile PBS. Blood was collected from the tail vein 3 h after FITC-dextran administration, and serum FITC-dextran was measured by fluorospectrometry (NanoDrop 3300; Thermo Scientific, Wilmington, DE, USA) with the excitation and emission wavelengths at 485 and 528 nm, respectively, using a standard curve of serially diluted FITC-dextran. Because spontaneous endotoxemia and serum BG elevation are associated with impaired GI permeability [18, 20], serum endotoxin and BG were measured by the HEK-Blue LPS Detection Kit 2 (InvivoGen, San Diego, CA, USA) and Fungitell assay (Associates of Cape Cod), respectively. The lower limit of detection of the endotoxin and Fungitell assays was 0.01 EU/mL and 7.8 pg/mL, respectively, and values <0.01 EU/mL and <7.8 pg/mL were recorded as 0.
Sample Analysis
Serum endotoxin and BG from human samples were measured as described above. Serum anti-dsDNA IgG in human samples was evaluated by ELISA assay (Euroimmun, Lübeck, Germany). In mouse samples, the detection of serum anti-dsDNA antibodies was performed following a previously published protocol [21]. Mouse urine was collected for 24 h using a metabolic cage (Hatteras Instrument, Cary, NC, USA) and urine protein was measured by Bradford protein assay. Kidney injury was determined by serum creatinine (QuantiChrom creatinine assay, DICT-500, BioAssay, Hayward, CA, USA). Serum cytokines (IL-6, TNF-α, and IL-10) were measured by ELISA assay (ReproTech, Oldwick, NJ, USA).
Induction of Macrophage Cytokine Production Protocol
A previously established protocol for the preparation of bone marrow-derived macrophages (BMs) was performed [22]. Anti-F4/80 and anti-CD11c antibody (BioLegend, San Diego, CA, USA) with flow cytometry was used for macrophage phenotype confirmation. Pachyman (a representative BG) at various doses with or without LPS (Escherichia coli 026:B6; Sigma-Aldrich) at a concentration of 10 ng/mL was incubated with macrophages (1 × 105 cells/well) in culture plates. Supernatants were then collected at specific time points and cytokine levels were measured by ELISA assay (ReproTech). The area under the curve (AUC) of cytokine responses after incubation with LPS alone or LPS with BG was calculated, in order to compare the degree of response between mouse strains.
Statistical Analysis
The data were presented as mean ± SE, and the statistical differences among groups were examined using the unpaired Student t test or one-way analysis of variance (ANOVA) with the Tukey comparison test for the analysis of experiments with 2 and 3 groups, respectively. Data at several time points were analyzed by repeated-measures ANOVA with the Bonferroni post hoc analysis. Survival analyses were evaluated with the log-rank test. p < 0.05 was considered statistically significant. SPSS v11.5 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis. In addition, the AUC of cytokine responses was calculated with GraphPad Prism v4.0 software and compared between the mouse strains with the unpaired Student t test.
Results
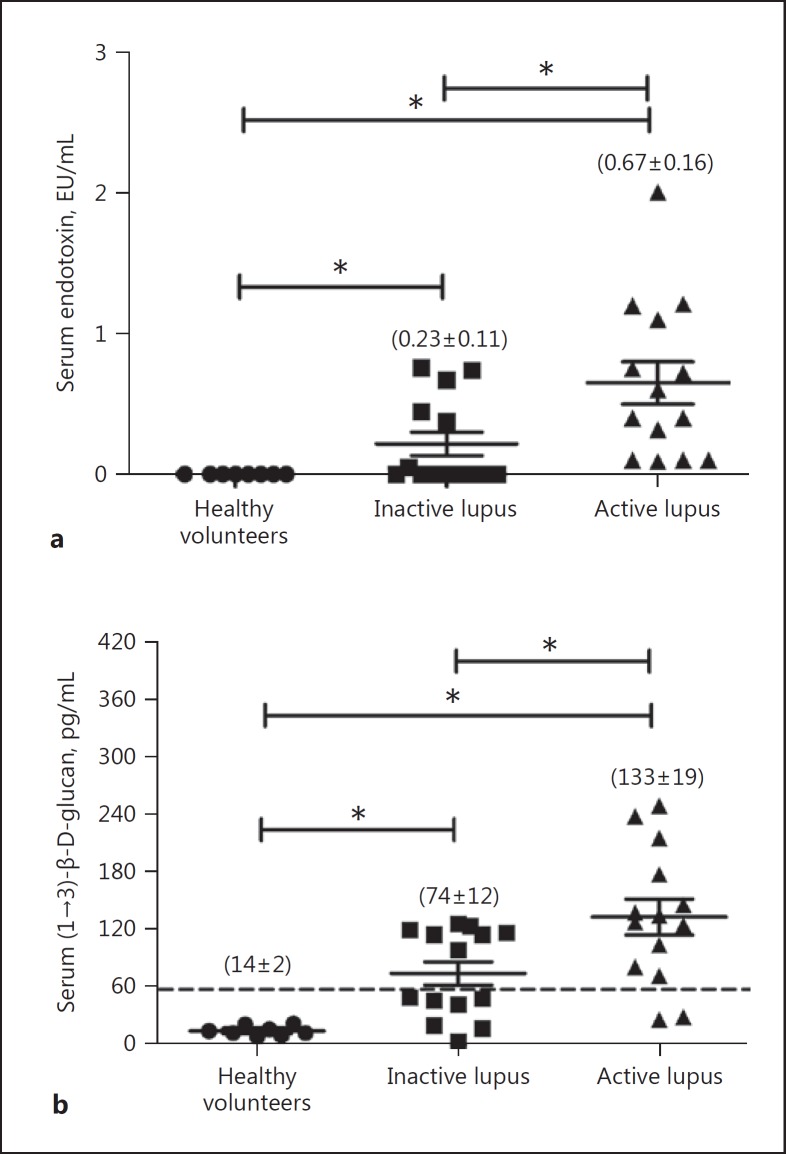
Spontaneous GI Leakage with Elevated Serum Endotoxin and BG in Patients with LN
The spontaneous presentation of endotoxin and BG in blood, without systemic infection, is indicative of GI permeability barrier impairment [18, 23]. Spontaneous endotoxemia and elevated serum BG (>60 pg/mL) were observed in most of the patients with active LN (Fig. 1). Interestingly, elevated serum BG was demonstrated in 86% (12 of 14 patients) and 50% (7 of 14 patients) of the cases with active and inactive LN, respectively (Fig. 1b), thereby supporting the hypothesis of gut leakage in lupus as previously described [3]. Serum endotoxin and BG level in healthy volunteers were very low, and patients with active LN showed a higher BG level than those with an inactive condition (Fig. 1a). Endotoxemia and β- glucanemia are observed in sepsis [18] and, given the elevated blood levels of endotoxin and BG in lupus patients, it is possible that endotoxin and BG might physiologically affect sepsis severity in the lupus context. Accordingly, the lupus mouse model was utilized to investigate this possibility.
Fig. 1.
A cross-sectional analysis demonstrated serum endotoxin (a) and serum (1→3)-β-D-glucan (b) titers in healthy volunteers (n = 8) and in patients with inactive (n = 14) and active lupus nephritis (n = 14) (see Materials and Methods). * p < 0.05.
Spontaneous GI Leakage with Elevated Serum Endotoxin and BG in 40-Week-Old FcGRIIb−/− Mice
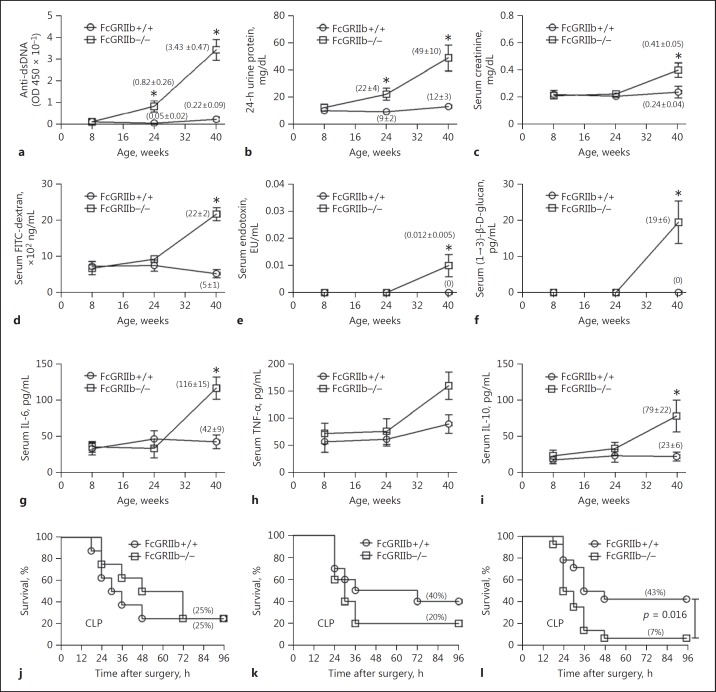
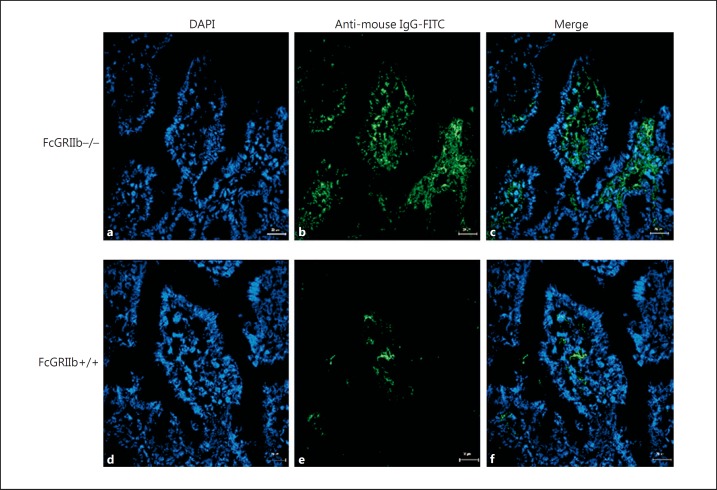
Immunological evidence and clinical manifestations of LN in mice were demonstrated by elevated anti- dsDNA and proteinuria with/without increased serum creatinine, respectively (Fig. 2a-c). Lupus manifestations, as age-related characteristics of FcGRIIb−/− mice, allowed the exploration of asymptomatic and symptomatic status in different age groups. Spontaneous GI leakage, determined by FITC-dextran assay and the spontaneous elevation of endotoxin and BG in the serum, was demonstrated in FcGRIIb−/− mice at 40 weeks, but not in younger age-groups or in wild-type mice (Fig. 2d-f). In parallel, serum levels of IL-6 and IL-10, but not of TNF-α, were higher in symptomatic 40-week-old lupus mice than in wild-type (Fig. 2g-i). In addition, immune complex deposition in the GI tract of symptomatic lupus mice was higher than in age-matched wild-type mice (Fig. 3), suggesting immune-induced gut injury. Immune complex deposition was demonstrated mostly at the area of the lamina propria of the mouse GI tract (Fig. 3).
Fig. 2.
Biological characteristics of FcGRIIb−/− mice, including anti-dsDNA (a), 24-h proteinuria (b), serum creatinine (c), gut leakage, as measured by FITC-dextran assay (d), endotoxemia (e), serum (1→3)-β-D-glucan (f), and spontaneous serum cytokines (g–i) in comparison with age-matched wild-type (FcGRIIb+/+) mice (n = 4–6) at each time point. Survival analyses of mice with cecal ligation and puncture (CLP)-induced sepsis in 8-week-old (j: n = 6/group), 24-week-old (k: n = 10/group), and 40-week-old mice (l: n = 14/group). * p < 0.05 versus FcGRIIb−/− at the same time point.
Fig. 3.
Representative immunofluorescence images from the jejunums of 40-week-old FcGRIIb−/− mice (a–c) versus wild-type controls (FcGRIIb+/+) (d–f). ×600. DAPI (blue) and goat anti-mouse IgG with FITC (green) were used for the identification of the nucleus and Fc portion of the immune complex deposition, respectively. The merged figures demonstrated that immune complex depositions were mostly found at the lamina propria of FcGRIIb−/− mice (c) when compared with wild-type mice (f).
To determine if there was age-dependent susceptibility to polymicrobial sepsis in FcGRIIb−/− mice, we induced sepsis with CLP surgery in different age groups. It was only in 40-week-old FcGRIIb−/− mice that we detected more severe sepsis than that in age-matched wild-type (Fig. 2j-l). Based on these results, we selected FcGRIIb−/− mice aged 8 and 40 weeks as representative of asymptomatic and symptomatic lupus, respectively, for the experiments.
GI Leakage Enhanced Sepsis Severity in Both 8-Week-Old and 40-Week-Old FcGRIIb−/− Lupus Mice
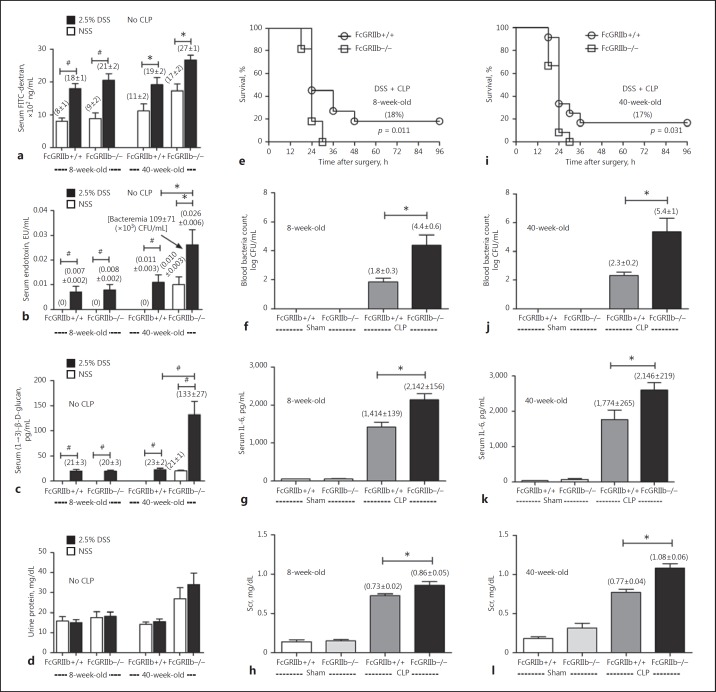
We hypothesized that the severe sepsis in symptomatic FcGRIIb−/− mice was, at least in part, due to GI leakage. Hence, GI leakage was induced with 1 week of DSS mixed into the drinking water, to explore the role of leaky gut in sepsis. Indeed, DSS induced mild gut leakage as demonstrated by elevated serum FITC-dextran, endotoxin, and BG in 8-week-old mice of both strains and in 40-week-old wild-type mice (Fig. 4a-c). Although gut leakage was already present in 40-week-old FcGRIIb−/− mice, DSS enhanced the severity, as demonstrated by all 3 of the parameters examined (Fig. 4a-c). Interestingly, spontaneous polymicrobial bacteremia presented only in 40-week-old FcGRIIb−/− mice with DSS (Fig. 4b). This implied that gut leakage is severe enough for the translocation of viable bacteria in 40-week-old FcGRIIb−/− mice with DSS insult. Of note, proteinuria was not enhanced by DSS administration (Fig. 4d).
Fig. 4.
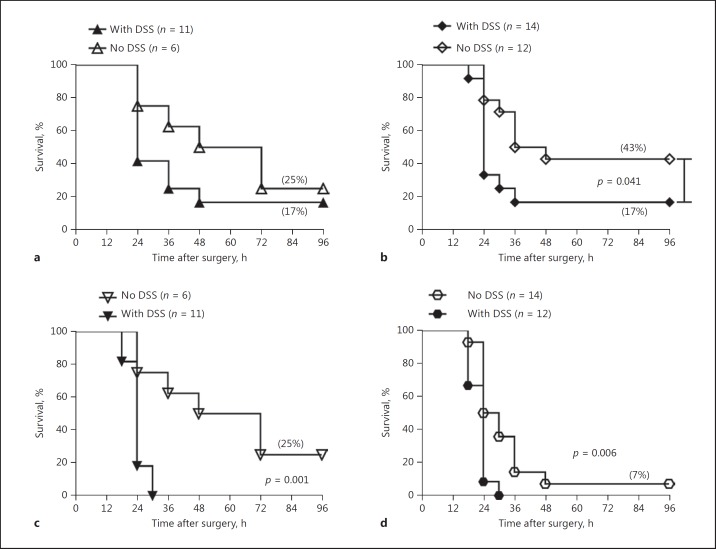
The characteristics of impaired gut permeability induced by 2.5% dextran sulfate solution (DSS), as measured by FITC-dextran assay (a), endotoxemia (b), serum (1→3)-β-D-glucan (c), and proteinuria (d) in 8-week-old (n = 11/group) and 40-week-old (n = 5–6/group) mice. Survival analysis of the severity of cecal ligation and puncture (CLP) sepsis after 2.5% DSS administration (e: n = 11/group; i: n = 12/group); other parameters at 18 h of CLP, including bacteremia (f, j), serum IL-6 (g, k), and serum creatinine (Scr) (h, l) were evaluated. * p < 0.05, #p < 0.001.
Subsequently, CLP surgery, performed in FcGRIIb−/− mice after 1 week of DSS administration, showed more severe sepsis than that in both 8-week-old and 40-week-old wild-type mice, as determined by survival rate, bacteremia, serum IL-6, and serum creatinine (Fig. 4e-l). All FcGRIIb−/− mice in both age groups died within 30 h after CLP with DSS (Fig. 4e, i). There was DSS- enhanced sepsis severity in 40-week-old wild-type mice, but the DSS-enhanced sepsis severity was greater in the FcGRIIb−/− mice from both age groups (Fig. 5).
Fig. 5.
Survival analyses of cecal ligation and puncture (CLP) in wild-type (FcGRIIb+/+; a, b) and FcGRIIb−/− (c, d) mice, with or without administration of 2.5% dextran sulfate solution (DSS) at 8 (a, c) and 40 (b, d) weeks of age.
Endotoxin Alone or in Combination with BG, but Not BG Alone, Enhanced Inflammatory Response and Sepsis Severity in FcGRIIb−/− Mice
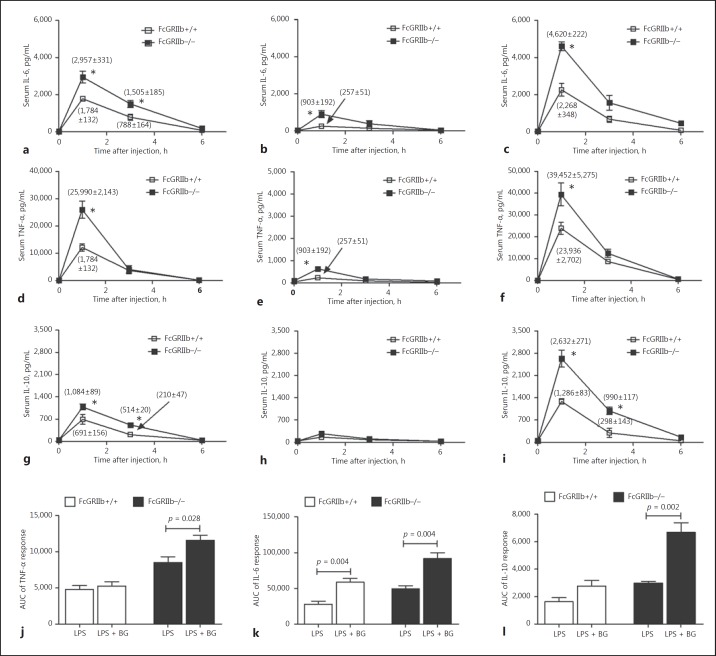
Endotoxemia was observed together with elevated serum BG in symptomatic FcGRIIb−/− mice. Hence, the physiological importance of both molecules, alone or in combination, was explored by administering them to asymptomatic 8-week-old FcGRIIb−/− mice. In the absence of sepsis induction, FcGRIIb−/− mice showed prominent serum cytokine responses after the administration of endotoxin and BG, both individually and in combination (Fig. 6a-i). The largest response occurred 1 h after the injection. Serum cytokine responses after BG alone were the lowest among these 3 conditions (Fig. 6b, e, h). Of note, BG at <5 mg/kg did not significantly induce serum cytokines in the mice of either strain (data not shown). Synergistic elicitation of cytokine responses was observed with BG and endotoxin, as determined from the AUC of the responses of all cytokines in the FcGRIIb−/− mice, but only IL-6 in the wild-type mice (Fig. 6j-l).
Fig. 6.
Serum cytokine responses of wild-type (FcGRIIb+/+) and FcGRIIb−/− mice (without cecal ligation and puncture) after intraperitoneal (i.p.) endotoxin (LPS, 1 mg/kg) injection with intravenous (i.v.) normal saline (NSS) (a, d, g), i.v (1→3)-β-D-glucan (BG, 50 mg/kg) with i.p. NSS (b, e, h), and i.p. LPS with i.v. BG (c, f, i). j–l The area under the curve (AUC) of serum cytokine responses in both mouse strains. * p < 0.05 versus FcGRIIb+/+ at the same time point.
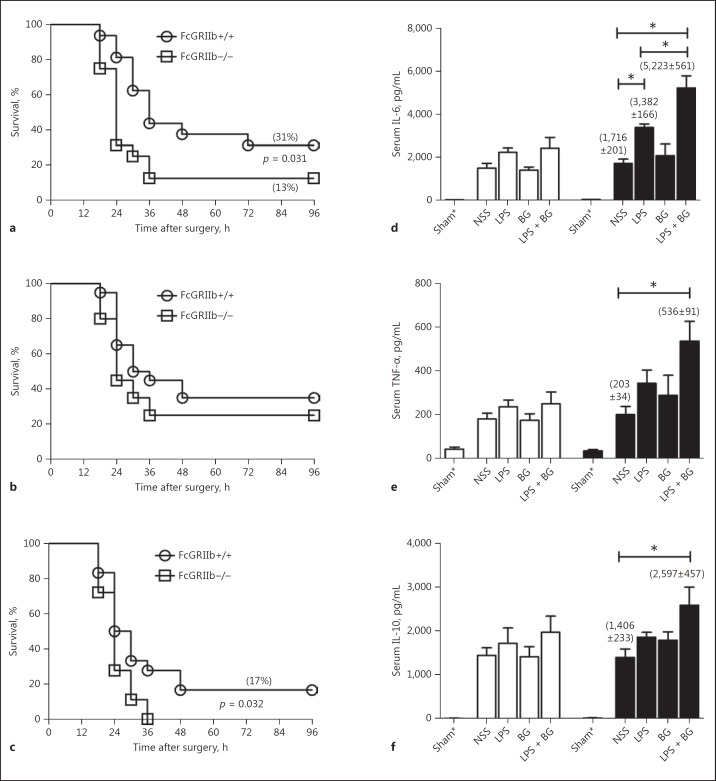
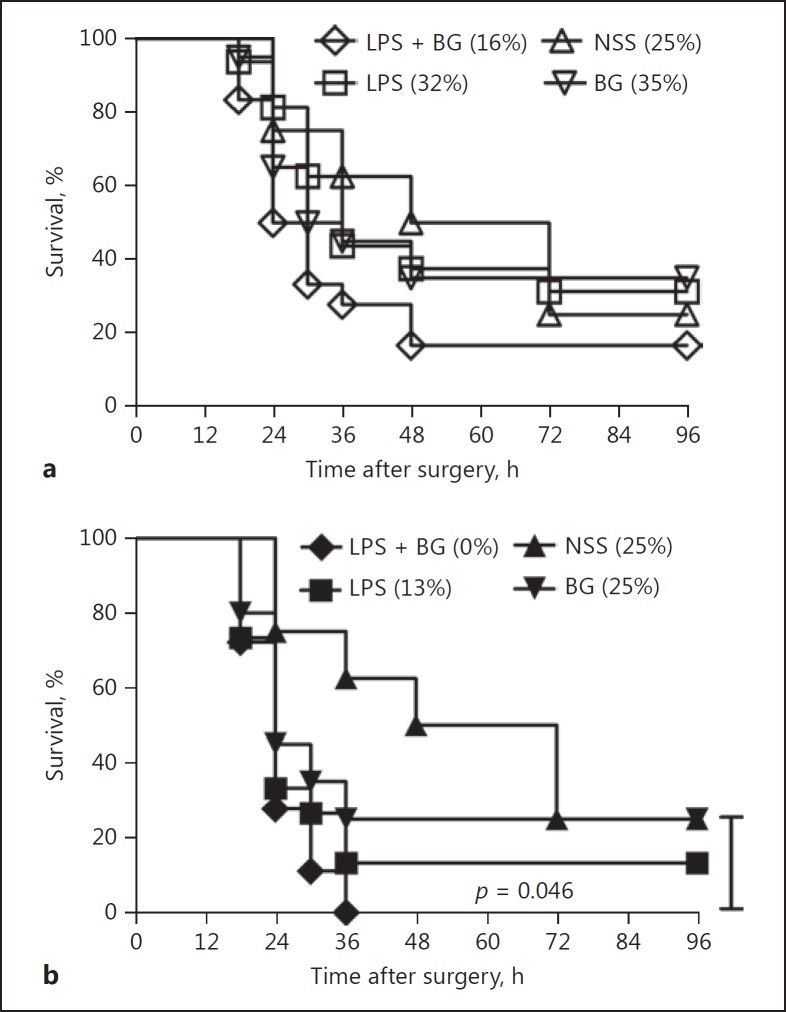
On the other hand, CLP and endotoxin administration, with or without BG (but not BG alone), enhanced sepsis severity, as determined by both survival and serum cytokine levels (Fig. 7). Sepsis severity was more prominent in FcGRIIb−/− than in wild-type mice (Fig. 7a-c). However, the synergy of BG with endotoxin, after the CLP of FcGRIIb−/− mice, was demonstrated only with serum TNF-α (Fig. 7d), not with IL-6 and IL-10 (Fig. 7e, f). Survival of CLP with NSS control or CLP with endotoxin or BG alone or endotoxin and BG in combination was not different in wild-type mice (Fig. 8a). In FcGRIIb−/− mice, the sepsis mortality of mice with endotoxin and BG was higher than in sepsis with BG but not different from sepsis with endotoxin (Fig. 8b).
Fig. 7.
Survival analyses of 8-week-old wild-type (FcGRIIb+/+) and FcGRIIb−/− mice with cecal ligation and puncture sepsis with intraperitoneal (i.p.) endotoxin (LPS) injection and intravenous (i.v.) normal saline (NSS) (a: n = 16/group), i.v. (1→3)-β-D-glucan (BG) with i.p. NSS (b: n = 20/group), and i.p. LPS with i.v. BG (LPS + BG) (c: n = 18/group). d–f Serum cytokine levels at 18 h after CLP surgery in these groups (n = 5–6/group). FcGRIIb+/+, white columns; FcGRIIb−/−, black columns.
Fig. 8.
Survival analyses of 8-week-old wild-type (FcGRIIb+/+) (a) and FcGRIIb−/− (b) mice with cecal ligation and puncture (CLP) sepsis with intraperitoneal (i.p.) and intravenous (i.v.) normal saline (NSS) (n = 8), i.v. NSS with i.p. endotoxin (LPS) (n = 16), i.v. (1→3)-β-D-glucan (BG) with i.p. NSS (n = 20), and i.v. BG with i.p. LPS (LPS + BG) (n = 18). (%), survival rate.
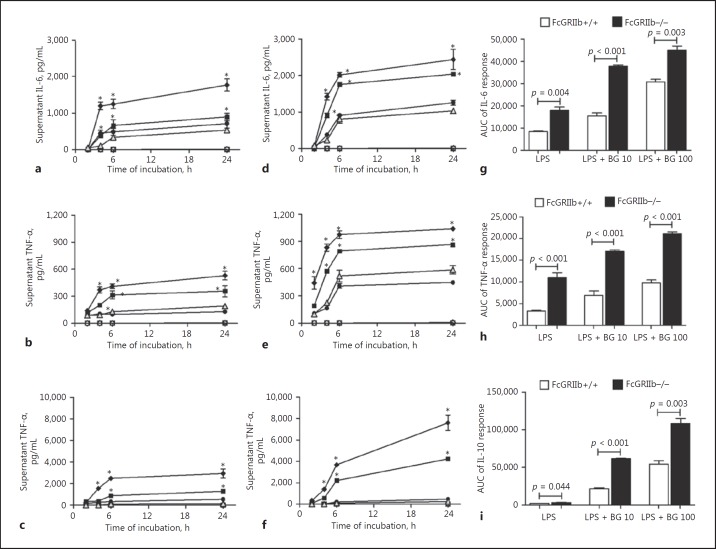
In order to see if there was a different strain-dependent synergistic effect of BG with endotoxin on immune cell inflammatory responses, BMs were tested. Interestingly, BG alone did not induce macrophage cytokine responses. But endotoxin (10 ng/mL) with BG (at 10 and 100 μg/mL, but not at 1 μg/mL) increased macrophage cytokine production (Fig. 9a-f). The AUC showed endotoxin synergy with BG at 10 and 100 μg/mL in FcGRIIb−/− and wild-type mice, and this was statistically significant (ANOVA with the Tukey comparison test, data not shown). In parallel with the in vivo results, endotoxin and BG synergy was more prominent in macrophages from FcGRIIb−/− mice than in those from wild-type mice, as demonstrated by the AUC of cytokine responses (Fig. 9g-i). These data suggest that, in FcGRIIb−/− mice, the enhanced synergistic elicitation of cytokines by coexposure to endotoxin and BG is due to the loss of inhibitory signaling.
Fig. 9.
Cytokine responses in macrophage culture supernatant from FcGRIIb−/− (a–c) or wild-type (FcGRIIb+/+) (d–f) mice after activation with (1→3)-β-D-glucan (BG) alone at different doses (100 μg/mL, ◇; 10 μg/mL, ◻; 1 μg/mL, ⚪), endotoxin (10 ng/mL LPS) alone (gray triangle), and BG + LPS (100 μg/mL BG + 10 ng/mL LPS, ◆; 10 μg/mL BG + 10 ng/mL LPS, ◼; 1 μg/mL BG + 10 ng/mL LPS, ⚫). Separate, triplicate experiments were performed. g–i The area under the curve (AUC) of cytokine responses in the macrophage culture supernatant (in vitro) from both strains with 10 ng/mL LPS alone and LPS with different doses of BG. BG 10, 10 μg/mL BG; BG 100, 100 μg/mL BG. * p < 0.05 versus LPS 10 ng/mL at the same time point.
Discussion
Spontaneous gut leakage and increased serum endotoxin/BG in symptomatic lupus demonstrate an impact upon sepsis severity. Prominent responses of FcGRIIb−/− mice and macrophages to endotoxin and BG in synergy, were demonstrated. These data support the potential importance of gut leakage in the enhancement of sepsis severity in the context of lupus.
Gut Leakage in LN
LN is a representative of a circulating immune complex (CIC)-mediated condition [24] and CIC gut-deposition is possible in active lupus due to the large GI surface area. Obvious GI manifestations of lupus are rare [25], but immune complex deposition in the gut [26] and spontaneous endotoxemia (an indirect indicator of gut leakage) can be observed in patients [3]. Interestingly, serum BG >60 pg/mL (a negative cut-off for invasive fungal infection) without fungal infection was demonstrated in most of our patients. The presence of elevated levels of endotoxin and BG, foreign molecules in mammals, suggested a GI permeability barrier defect. As the direct measurement of gut leakage in patients was difficult, we evaluated it in FcGRIIb−/− mice by FITC-dextran translocationand immune complex deposition in the gut tissue of symptomatic lupus mice. These were observed in 40-week-old FcGRIIb−/− mice but not in the younger age groups. In 40-week-old FcGRIIb−/− mice, gut leakage was observed, together with the more severe proteinuria and renal injury.
The Influence of Impaired GI Barrier and Gut Translocation of Endotoxin and BG on Sepsis in FcGRIIb−/− Mice
Gut leakage induced by DSS caused spontaneous endotoxemia and elevated serum BG in wild-type mice and in asymptomatic lupus mice (8-week-old FcGRIIb−/− mice; nonspontaneous gut leakage). Interestingly, without DSS, the severity of CLP was comparable between both mouse strains. With DSS, CLP sepsis severity was enhanced only in 40-week-old wild-type mice, but in both age groups of the FcGRIIb−/− mice. DSS had a greater impact on sepsis severity in FcGRIIb−/− mice than in wild-type mice. As gram-negative bacteria and fungi are prominent constituents of the normal microbiota in the gut [4, 27], translocated LPS and BG, as major cell wall components of these organisms, acted synergistically, but affected FcGRIIb−/− mice more than wild-type mice. This may have been due to the hyperimmune responses of FcGRIIb−/− mice, i.e., due to defective regulatory signaling [10]. Consistent with this hypothesis, the administration of LPS or BG, individually or in combination, without CLP sepsis, induced higher serum cytokine responses in FcGRIIb−/− mice than in wild-type mice. Although BG alone induced a mild increase in serum cytokines, BG showed a synergistic effect with endotoxin. To determine if there was synergy in sepsis, combinatorial LPS and/or BG administration was conducted with CLP surgery in 8-week-old mice. The administration of LPS with or without BG enhanced sepsis severity to a greater degree in FcGRIIb−/− mice than in wild-type mice.
In parallel, the incubation of endotoxin alone or with BG, but not BG alone, induced greater macrophage cytokine production. This supports the endotoxin-BG synergy observed in several previous studies [19, 28]. However, BG alone could not induce macrophage cytokine production in either FcGRIIb−/− or wild-type mice, suggesting a limited independent proinflammatory potential [29, 30]. The main pattern recognition receptors responsible for the detection of endotoxin and BG are TLR-4 and dectin-1, respectively [31]. Their simultaneous activation by BG, together with endotoxin, leads to enhanced macrophage cytokine responses [32]. The defect in negative signaling of FcGRIIb−/− macrophages was likely responsible for the more prominent synergistic responses when compared with the wild-type mice.
Taken together, our data support the hypothesis that (i) spontaneous gut leakage may present in lupus without obvious GI manifestations, (ii) gut translocation of endotoxin and BG are associated with an increased inflammatory state, and (iii) endotoxin and BG, in synergy, enhance sepsis severity but are more acute in FcGRIIb−/− lupus mice. Regarding translational aspects, strategies to attenuate or identify gut leakage and/or elevated serum endotoxin (and BG) in patients with lupus might be beneficial. Additional studies will be required to evaluate the role of gut luminal content translocation in this model of autoimmune disease.
Disclosure Statement
The authors state no conflict of interest.
Author Contributions
J.I-A., S.S., and A.L. designed experiments. J.I-A., S.S., N.W., A.T., and A.L. performed experiments. J.I-A., M.F., and A.L. prepared the manuscript. All authors discussed the results and commented on the manuscript.
Acknowledgements
This work was supported by funding from the Thai government (GB-B_61_071_30_29), a grant for International Research Integration, Chula Research Scholar, Ratchadaphisek Somphot Endowment Fund (2017), the Chulalongkorn University (760001-HR), and an International Research Network Thailand Research Fund (IRN59W0004).
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Campbell AW. Autoimmunity and the gut. Autoimmune Dis. 2014;2014:152428. doi: 10.1155/2014/152428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, Maurer K, Costa Reis P, Song L, Petri M, Sullivan KE. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One. 2014;9:e93846. doi: 10.1371/journal.pone.0093846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Worasilchai N, Finkelman M, Chindamporn A, Palaga T, Tumwasorn S, Leelahavanichkul A. Gastrointestinal colonization of candida albicans increases serum (1->3)-β-D-glucan, without candidemia, and worsens cecal ligation and puncture sepsis in murine model. Shock. 2018;49:62–70. doi: 10.1097/SHK.0000000000000896. [DOI] [PubMed] [Google Scholar]
- 6.Ropes MW. Observations on the natural course of disseminated lupus erythematosus. Medicine (Baltimore) 1964;43:387–391. doi: 10.1097/00005792-196405000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38:473–485. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya N, Kyogoku C. Role of Fc gamma receptor IIb polymorphism in the genetic background of systemic lupus erythematosus: insights from Asia. Autoimmunity. 2005;38:347–352. doi: 10.1080/08916930500123926. [DOI] [PubMed] [Google Scholar]
- 9.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIb-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 10.Clatworthy MR, Willcocks L, Urban B, Langhorne J, Williams TN, Peshu N, Watkins NA, Floto RA, Smith KG. Systemic lupus erythematosus-associated defects in the inhibitory receptor Fcgamma RIIb reduce susceptibility to malaria. Proc Natl Acad Sci USA. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ondee T, Surawut S, Taratummarat S, Hirankan N, Palaga T, Pisitkun P, Pisitkun T, Leelahavanichkul A. FC gamma receptor IIb deficient mice: a lupus model with increased endotoxin tolerance-related sepsis susceptibility. Shock. 2017;47:743–752. doi: 10.1097/SHK.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 12.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc gamma RIIb-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 13.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in Fc gamma RIIb−/− mice. J Exp Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 15.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 16.Leelahavanichkul A, Somparn P, Bootprapan T, Tu H, Tangtanatakul P, Nuengjumnong R, Worasilchai N, Tiranathanagul K, Eiam-Ong S, Levine M, Chinampon A, Srisawat N. High-dose ascorbate with low-dose amphotericin B attenuates severity of disease in a model of re-appearance of candidemia during sepsis in the mouse. Am J Physiol Regul Integr Comp Physiol. 2015;309:R223–234. doi: 10.1152/ajpregu.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leelahavanichkul A, Somparn P, Issara-Amphorn J, Eiam-ong S, Avihingsanon Y, Hirankarn N, Srisawat N. Serum neutrophil gelatinase associated lipocalin (NGAL) outperforms serum creatinine in detecting sepsis-induced acute kidney injury, experiments on bilateral nephrectomy and bilateral ureter obstruction mouse models. Shock. 2016;45:570–576. doi: 10.1097/SHK.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 18.Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, Tachaboon S, Srisawat N, Finkelman M, Chindamporn A. Gastrointestinal leakage detected by serum (1->3)-β-D-glucan in mouse models and a pilot study in patients with sepsis. Shock. 2016;46:506–518. doi: 10.1097/SHK.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 19.Kikkert R, Bulder I, de Groot ER, Aarden LA, Finkelman MA. Potentiation of Toll-like receptor-induced cytokine production by (1->3)-β-D-glucans: implications for the monocyte activation test. J Endotoxin Res. 2007;13:140–149. doi: 10.1177/0968051907080024. [DOI] [PubMed] [Google Scholar]
- 20.Derikx JP, Luyer MD, Heineman E, Buurman WA. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol. 2010;16:5272–5279. doi: 10.3748/wjg.v16.i42.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A, Gu Y, Davidson A. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106:91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonyatecha N, Sangphech N, Wongchana W, Kueanjinda P, Palaga T. Involvement of Notch signaling pathway in regulating IL-12 expression via c-Rel in activated macrophages. Mol Immunol. 2012;51:255–262. doi: 10.1016/j.molimm.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponticelli C, Salvadori M, Coppo R. The kidney, a victim and culprit of autoimmune and alloimmune responses. Nephron Clin Pract. 2011;119:c200–c204. doi: 10.1159/000328913. [DOI] [PubMed] [Google Scholar]
- 25.Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J Gastroenterol. 2010;16:2971–2977. doi: 10.3748/wjg.v16.i24.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brentjens JR, Andres GA. The pathogenesis of extrarenal lesions in systemic lupus erythematosus. Arthritis Rheum. 1982;25:880–886. doi: 10.1002/art.1780250733. [DOI] [PubMed] [Google Scholar]
- 27.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engstad CS, Engstad RE, Olsen JO, Osterud B. The effect of soluble β-1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int Immunopharmacol. 2002;2:1585–1597. doi: 10.1016/s1567-5769(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez M, Domingo E, Municio C, Alvarez Y, Hugo E, Fernandez N, Sanchez Crespo M. Polarization of the innate immune response by prostaglandin E2: a puzzle of receptors and signals. Mol Pharmacol. 2014;85:187–197. doi: 10.1124/mol.113.089573. [DOI] [PubMed] [Google Scholar]
- 30.Municio C, Alvarez Y, Montero O, Hugo E, Rodriguez M, Domingo E, Alonso S, Fernandez N, Crespo MS. The response of human macrophages to beta-glucans depends on the inflammatory milieu. PLoS One. 2013;8:e62016. doi: 10.1371/journal.pone.0062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]