Abstract
Spleen tyrosine kinase (SYK) is a nonreceptor tyrosine kinase which associates directly with extracellular receptors, and is critically involved in signal transduction pathways in a variety of cell types for the regulation of cellular responses. SYK is expressed ubiquitously in immune and nonimmune cells, and has a much wider biological role than previously recognized. Several studies have highlighted SYK as a key player in the pathogenesis of a multitude of diseases. Pseudomonas aeruginosa is an opportunistic gram-negative pathogen, which is responsible for systemic infections in immunocompromised individuals, accounting for a major cause of severe chronic lung infection in cystic fibrosis patients and subsequently resulting in a progressive deterioration of lung function. Inhibition of SYK activity has been explored as a therapeutic option in several allergic disorders, autoimmune diseases, and hematological malignancies. This review focuses on SYK as a therapeutic target, and describes the possibility of how current knowledge could be translated for therapeutic purposes to regulate the immune response to the opportunistic pathogen P. aeruginosa.
Keywords: Pseudomonas aeruginosa, Infection, Cystic fibrosis, Inflammation, Spleen tyrosine kinase, Small molecule inhibitor, Cystic fibrosis transmembrane conductance regulator
Introduction
Spleen tyrosine kinase (SYK) is a nonreceptor tyrosine kinase involved in signal transduction in a variety of cell types; it associates with different receptors on the surface of various cells such as B cells, mast cells, monocytes, macrophages, and neutrophils, and even osteoclasts and breast cancer cells. Following the engagement of these receptors with their ligands, SYK is activated and orchestrates diverse cellular responses, including cytokine production (in T cells and monocytes) and phagocytosis (in macrophages) [1, 2]. SYK is expressed ubiquitously in both hematopoietic [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] and nonhematopoietic cells [15, 16, 17, 18, 19, 20]. Notably, this widespread expression of SYK in human tissues implies that it plays important roles in different organs. Importantly, SYK is expressed in lung epithelial cells [21, 22], which are the major components of the airway lining and the site of infection by Pseudomonas aeruginosa. The role of SYK in these structural cells is puzzling, but recent studies have shed some light on it. For these reasons, it may represent an attractive target for a new therapeutic strategy for treating P. aeruginosa infection using the inhibition of SYK. In this review, we discuss the role of SYK and the effect of SYK inhibitors in the treatment of P. aeruginosa infection.
Structural Basis of SYK Activation
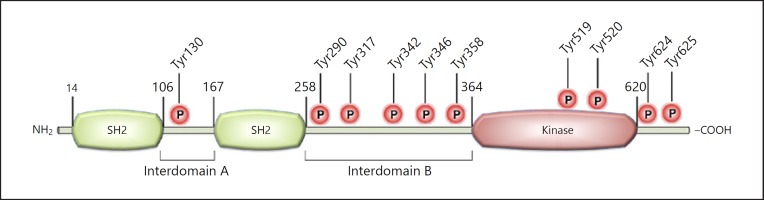
SYK, a 72-kDa protein, is composed of 2 SRC homology (SH2) and 1 kinase domains, with interdomain A located between the 2 SH2 domains and interdomain B between the SH2 and kinase domains; these interdomains contain linker tyrosines, which can undergo phosphorylation (Fig. 1) [22, 23, 24, 25]. SYK contains at least 10 tyrosine residues that can be autophosphorylated, and thus provide binding sites for other molecules bearing SH2 domains [26]. Due to its catalytic activity and the ability to bind other proteins via the interaction between phosphorylated tyrosines and SH2 domains, SYK has both kinase and adaptor protein properties.
Fig. 1.
Structure of SYK protein: a schematic diagram of the linear structure of SYK with the tyrosines marked that are phosphorylated after activation.
There are 3 states of SYK: inhibition of the kinase, activated kinase via phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs), and activated kinase via phosphorylation of linker tyrosines. In the inhibited kinase state, the binding occurs between interdomain A, interdomain B, and the kinase domain, producing the stable configuration of SYK; breaking apart this arrangement allows for the activation of the protein kinase to occur [24]. ITAM tyrosine residues are rapidly phosphorylated following the engagement of classical immunoreceptors, i.e., B cell receptors (BCRs), T cell receptors (TCRs), and Fc receptors (FcRs), and leading to the recruitment and activation of SYK. The other state of SYK is the activation of the kinase through autophosphorylation of the linker tyrosines in the interdomains; this process does not involve dependence on the phosphorylated ITAMs for activation [22, 23, 24, 25]. SYK can sustain activation following the temporary interaction with phosphorylated ITAMs by means of autophosphorylation of the linker tyrosines [24].
SYK activation is not restricted to the 2 mechanisms stated above; studies have also shown that SYK mediates signaling via classes of receptors including integrin, G protein-coupled, and C-type lectins that do not contain conventional ITAMs [22, 27]. During an inflammatory response of the immune cells via a result of a variety of different signaling pathways, cytokines are produced as well; studies have shown that cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, produced during inflammation also have the ability to activate SYK by means of cytokine signaling [27]. Collectively, these studies have dramatically changed our view of SYK.
SYK and Innate Immunity
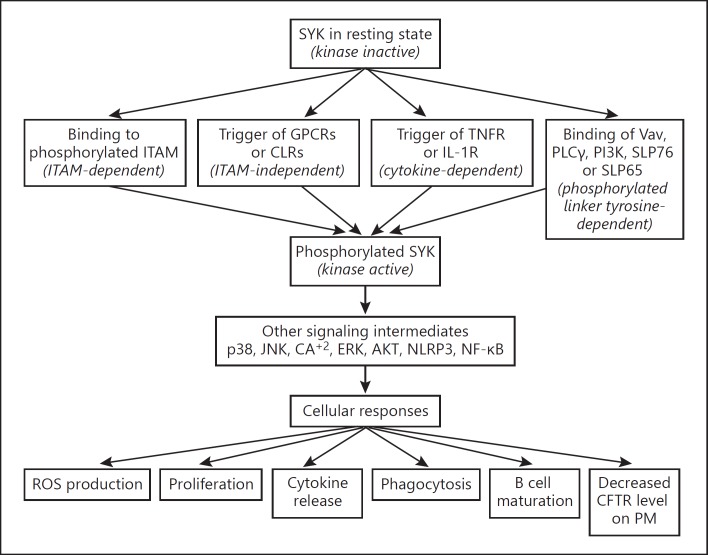
The innate immune system plays a leading role, through the cooperation of different germline-encoded pattern recognition receptors (PRRs), in detecting both pathogen- and damage-associated molecular patterns (PAMPs and DAMPs, respectively) and triggering immune responses. Studies have shown that many PPRs participate in the immune response to P. aeruginosa infection, such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), etc. [28, 29]. Recently, SYK has been found to be a vital component of these pathways, which play a crucial role in the innate immune response including pathogen recognition, inflammasome activation, and even antifungal defense [24, 30, 31]. Following the activation of the kinase, SYK-mediated downstream signaling occurs as a result. SYK can bind directly to 4 binding partners: Vav, phospholipase Cγ (PLCγ), phosphoinositide 3-kinase (PI3K), and the SH2 domain of leukocyte proteins 76 or 65 (SLP76 or SLP65, respectively). These 4 binding partners will further activate downstream signaling components and lead to an eventual change in cellular response. Such cellular responses include reactive oxygen species (ROS) production, cell proliferation, cytokine release, and inflammatory responses (Fig. 2) [24]. Presently, there is little research on the involvement of SYK in cellular responses to P. aeruginosa infection or targeting SYK for protecting infected human cells against the deleterious effects associated with this infection. However, it has been demonstrated in several allergic disorders, autoimmune diseases, hematological malignancies, and innate antifungal immunity.
Fig. 2.
General mechanism of SYK activation and SYK-mediated signaling: a pathway demonstrating the involving of downsignaling associated with SYK activation. AKT, protein kinase B; ERK, extracellular signal-regulated kinase; GPCRs, G protein-coupled receptors; IL-1R, interleukin-1 receptor; JNK, c-Jun N-terminal kinase; PM, plasma membrane; TNFR, tumor necrosis factor receptor.
It is well established that SYK activation in leukocytes is essential for phagocytosis and the development of B- and T-lymphocytes [24]. Studies have shown that many CLRs, such as Dectin-1 (also known as CleC7A) and Mincle (also known as CleC4e), can resist fungi, mainly by activating the downstream SYK/caspase recruitment domain-containing protein 9 (CARD9)/nuclear factor (NF)-κB signaling pathway [32, 33, 34, 35, 36]. Recent studies have revealed the importance of SYK during fungal infection by Aspergillus fumigatus [37]. Researchers have proved that SYK associates with invasive breast cancer [38] and is closely related to the occurrence and development of digestive tract tumors [39].
As SYK is positioned upstream in the cell-signaling pathway, therapies targeting SYK might be more advantageous than inhibiting a single downstream event [40]. These make SYK a therapeutic target for an array of inflammatory diseases. For this reason, many pharmaceutical companies and academic institutions have been involved in the development of small molecule SYK inhibitors. Recent studies have demonstrated the ability of SYK to regulate the production of proinflammatory molecules by bronchial epithelial and monocytic cells, which are stimulated with TNF-α, rhinovirus, or P. aeruginosa [25, 27, 30, 31, 41]. For these reasons, SYK may represent an attractive target for a new therapeutic strategy of treating P. aeruginosa infection by inhibiting SYK kinase. Indeed, several studies have highlighted SYK as a key player in the pathogenesis of a multitude of diseases [2, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51]. Several pathologies can be treated through the inhibition of SYK activity. Indeed, there is great interest in the field of more selective commercially available small molecule SYK inhibitors [52].
SYK and Cystic Fibrosis
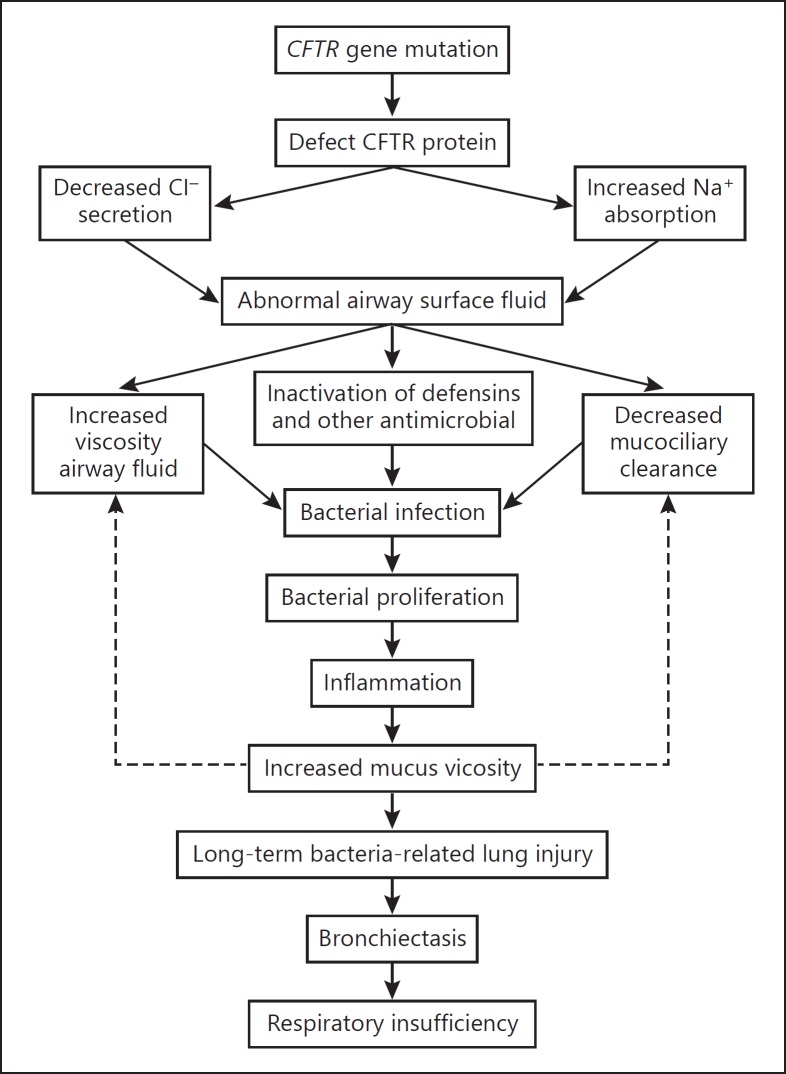
Cystic fibrosis (CF) is an autosomal recessive disease, mainly occurring in the Caucasian population. The condition is the manifestation of mutations in a transmembrane protein called cystic fibrosis transmembrane conductance regulator (CFTR), which commonly results in a loss of the protein or deficiency of its function [53, 54]. Mostly, CFTR functions as a chloride ion (Cl–) channel at the apical surface of secretory epithelia. CFTR is a member of the ATP-binding cassette transporter family, which hydrolyzes ATP to pump substrates, such as ions, vitamins, drugs, toxins, and peptides across biological membranes (Fig. 3) [55]. CF is therefore considered as a disease of ion transport across the epithelium that affects ion balance in the epithelium of the respiratory tract [56]. The most significant effect of CFTR mutation is the defect of ciliary clearance that results in the accumulation of mucus in the lung, creating an optimal environment for bacteria. Moreover, the elevated levels of sodium chloride in airway secretions severely weaken the host pulmonary innate defenses [57, 58].
Fig. 3.
Effect of altered CFTR function in cystic fibrosis (CF) and promotion of chronic pulmonary infection. Defective ion exchanges in the airway epithelium lead to bacterial colonization and inflammation in the lungs of CF patients.
Since the discovery of CFTR in 1989, many mutations in the gene have been identified; approximately 127 have been confirmed as causing the disease CF [59]. Among these mutations, a phenylalanine (3-bp) deletion at position 508 in the polypeptide chain (ΔF508) results in a protein that fails to mature properly and becomes degraded [55, 60]. ΔF508 is present in nearly 85% of CF patients in at least 1 allele. A connection has been made between mutant or missing CFTR in human lung epithelial cell membranes and the failure of innate immunity, which can lead to the initiation of P. aeruginosa infection. One study indicated that human cells use CFTR as a receptor for the internalization of P. aeruginosa via endocytosis, and the subsequent removal of bacteria from the airway that does not occur in the absence of functional CFTR, resulting in an increased bacterial load in the lungs [61]. Conversely, data from another study showed that peripheral blood mononuclear cells (PBMCs) derived from CF individuals display preserved inflammatory responses in response to P. aeruginosa infection versus PBMCs from healthy control individuals [62]. The study also showed that CFTR dysfunction did not alter IL-1β production when it compared the release of this cytokine from THP-1 human monocytic cells and PBMCs from CF and healthy control subjects following a prior treatment with a CFTR inhibitor. The static mucosal environment is presumed to render individuals susceptible to opportunistic infections. CF patients become infected (to some extent in an age-related pattern) by multiple microorganisms, specifically Haemophilus influenzae, Staphylococcus aureus, the Burkholderia cepacia complex, and a high proportion (as many as 80% of adult CF patients) are infected with P. aeruginosa [63]. As a result of its physiological properties, pattern of gene expression, and antibiotic resistance, which cause it to grow in biofilms that are significantly different from planktonic cultures, P. aeruginosa is challenging to treat [64, 65]. This persistent bacterial infection underlies the chronic lung inflammation that CF patients experience. Understanding the changes in the innate immune mechanisms in the lungs, a result of dysfunctional CFTR and persistent P. aeruginosa infection, is paramount to changing the natural course of CF disease.
The number of CFTR protein copies on the plasma membrane results from a balance between anterograde trafficking (i.e., CFTR is delivered from the endoplasmic reticulum to the plasma membrane), endocytosis (a process through which CFTR is retrieved from the membrane into vesicles), and recycling (with the return of the internalized CFTR to the plasma membrane). Remarkably, 1 of the protein kinases involved in CFTR trafficking is SYK. This nonreceptor tyrosine kinase has been reported to phosphorylate CFTR, leading to decreased levels of CFTR in the plasma membrane [66, 67]. This role of SYK in regulating protein trafficking has been reported previously for other substrates, e.g., trafficking a resident of the trans-Golgi network (TGN) 38 [68], the engaged high-affinity IgE receptor (FcεRI) [69], and the small GTPase Rac1 [70] (shown to play a role in CFTR trafficking and membrane anchoring [71]). Recent findings have shown that phosphorylation of CFTR by SYK results in reducing the abundance of CFTR in the plasma membrane [72]. Importantly, SYK-associated CFTR phosphorylation might not be a major determinant in CF patients. CF individuals have a defective CFTR as a result of misfolding, consequent degradation, altered expression, or preventing the translation of this specific protein, all of which lead to very low levels of CFTR [73, 74, 75, 76]. SYK knockdown in airway epithelial cells downregulates proinflammatory mediators, such as IL-6 and intercellular adhesion molecule (ICAM)-1 [22], both elevated in CF patients [77]. Recent studies expanded our understanding to recognize SYK as a potential target to attenuate the proinflammatory mediators in P. aeruginosa-infected CF patients.
Innate Immune Response to P. aeruginosa Infection
P. aeruginosa causes systemic life-threating infection in immunocompromised individuals and chronic lung infection in CF patients. The major determinant of morbidity and mortality in CF patients can be attributed to the progressive deterioration of lung function resulting from chronic infection by such a ubiquitous opportunistic pathogen as P. aeruginosa [30, 78]. During the infectious process, P. aeruginosa provokes a potent inflammatory response of infected tissue characterized by the activation of transcription factors, NF-κB, and activator protein 1 (AP-1). This results in the release of proinflammatory mediators, i.e., cytokines (TNF-α, IL-1β, and IL-6), chemokines (IL-8 and regulated on activation normal T cell-expressed and secreted [RANTES]), an increase in the expression of adhesion molecules (ICAM-1), the release of ROS, the recruitment of activated neutrophils, and severe damage to the tissues, eventually causing lung failure [79]. The infection of the airways by P. aeruginosa is accompanied by the activation of proinflammatory intracellular signaling pathways [80]. The activation of intracellular protein kinases has a significant role in the pathogenesis of P. aeruginosa lung infection. It has been demonstrated that the bacterial invasion and cytotoxic effect of P. aeruginosa, as well as the hyperproduction of IL-8 and mucin by infected lung epithelial cells, depend on the activation of the p38 and ERK1/2 mitogen-activated protein kinase (MAPK) signaling cascade and the Src-like tyrosine kinases p60Src, p59Fyn, and Lyn [81, 82, 83, 84].
Airway inflammation is a dominant pathophysiological characteristic of P. aeruginosa infection, influencing both the severity of the disease and its outcomes. In addition, P. aeruginosa is intrinsically resistant to many antibiotics, making treatment difficult and often unsuccessful [85]. Based on the rapidly growing understanding of intracellular signaling pathways involved in the pathogenesis of bacterial inflammation, targeting the inhibition of specific signaling pathways/molecules is a potential treatment strategy for P. aeruginosa lung infection.
Effect of SYK Inhibitor in P. aeruginosa Infection
The potent signaling abilities of SYK are due to both its molecular structure and its strategic localization in the proximal part of intracellular signaling cascades. Considering the vital role of inflammation in the pathogenesis of P. aeruginosa lung infection, the downregulation of proinflammatory signaling pathways via an SYK inhibitor may be a beneficial addition to the antibacterial therapy of such conditions. Studies have found that the natural SYK inhibitor piceatannol can inhibit the essential mechanisms of P. aeruginosa pathogenesis, i.e., bacterial internalization, production of proinflammatory mediators, oxidative stress, and apoptosis of infected human airway epithelial cells [30], all of which indicate that SYK is involved in the regulation of inflammatory responses caused by P. aeruginosa. Other studies, using a model of human monocytic cells, found that a small molecule inhibitor, R406, decreased both the inflammatory responses and the apoptosis induced by P. aeruginosa infection [31].
SYK has been recently identified as a crucial mediator of NLRP3 inflammasome activation and IL-1β secretion in macrophages stimulated with fungi and crystals [86]. Although the underlying molecular mechanisms are still being defined, SYK is known to regulate ROS production and lysosomal activity, 2 significant signals for NLRP3 inflammasome activation in macrophages [24]. It was recently found that inhibition of SYK reduced the release of bioactive IL-1β by macrophage cells infected by P. aeruginosa [31]. This suggests that SYK may regulate innate immune responses to P. aeruginosa via its involvement in inflammasome activation. Other studies have shown that the inhibition of SYK activity might be effective to modulate NLRP3 activation [87].
The release of the proinflammatory cytokine IL-1β results in the recruitment of effector cell populations of the immune response and tissue repair for host defense against infected pathogens [88]. Moreover, uncontrolled, excessive, or prolonged production of IL-1β may cause tissue damage, which can eventually interfere with pathogen clearance. Excessive IL-1β production is causally associated with the activation of a variety of inflammasomes, e.g., NLRP3 which receives a lot of biomedical attention [88], which are discussed elsewhere [89]. In addition, SYK and the NLRP3 inflammasome are key regulators of fungus-induced IL-1β production [90, 91, 92, 93]. As it has been demonstrated that fungal infection stimulates NLRP3 through a pathway requiring SYK activation, inhibiting SYK may potentially be beneficial in cases of potent inflammatory responses. Indeed, the identification of this cross-talk between SYK and inflammasomes might also be involved in P. aeruginosa infection [31].
The role of SYK in the regulation of inflammasome activation and ROS production induced by P. aeruginosa infection of human cells needs to be addressed to clarify the mechanisms behind the involvement of SYK-mediated signaling in the regulation of innate immune responses to P. aeruginosa infection. Based on the literature, studies suggest an association of SYK and the regulation of innate immune and inflammatory responses to P. aeruginosa, endorsing that SYK mediates inflammasome activation and promotes enhanced production of proinflammatory mediators by infected cells. Indeed, a significant decrease in the release of proinflammatory mediators by both P. aeruginosa-infected human macrophages (IL-1β and TNF-α) and lung epithelial cells (TNF-α) following SYK inhibition by R406 has been reported recently [31].
Potential Complications Associated with SYK Inhibition
In this review, SYK as an anti-inflammatory therapy in combination with antibiotics has been considered for the treatment of diseases associated with P. aeruginosa infections, which are characterized by strong inflammation of infected tissues. Despite the encouraging results of SYK inhibition as a valuable therapy, some potential obstacles are still associated with the use of SYK inhibitors. SYK is required for Fc-receptor-mediated phagocytosis, antigen presentation, and the maturation and survival of dendritic cells and B- and T-lymphocyte lineages [94, 95].
Blocking SYK signaling could therefore be particularly problematic in the context of immune cells. Studies showed that neutrophils lacking SYK reduce the host defense against bacterial infection [96]. The macrophages and neutrophils from CF patients, in particular, are already quite dysfunctional and have many abnormal signaling pathways; this impairs phagocytes, intracellular killing, and cellular migration [97]. Thus, there is a very fine balance to be maintained between damping the proinflammatory response and preserving the host defense against infected pathogens. It should be noted that a clinical trial of BILL 284 BS, an LTB4 receptor antagonist, was terminated due to an increase in serious adverse pulmonary-related events and P. aeruginosa bacteremia [98, 99]. More small molecule SYK inhibitors, along with their side effects, are discussed elsewhere [100, 101, 102, 103, 104].
A number of alternative approaches to reducing the inflammatory responses associated with pulmonary exacerbation in CF patients have been studied [105]. Several therapies targeting general inflammatory pathways in CF have not been successful. The use of SYK as a targeted anti-inflammatory could progress to immune suppression. It is noteworthy that CF patients are quite susceptible to fungal infections and so SYK inhibition could be detrimental to their health. Moreover, no long-term treatment with SYK inhibitors has yet been demonstrated. Addressing these possibilities of SYK-associated complications will be interesting as an anti-inflammatory approach. More studies are needed to understand the consequences of SYK inhibition, especially with persistent infection by P. aeruginosa in CF patients which leads to irreversible lung destruction [106].
Concluding Remarks
P. aeruginosa can cause chronic lung infection and systemic life-threating diseases in CF patients and immunocompromised individuals. Based on the literature, SYK mediates innate the immune response to P. aeruginosa infection. SYK is already considered a potential target of anti-inflammatory therapy for various clinical conditions. Indeed, SYK is what mostly controls the inflammatory process, and so the inhibition of SYK activity might prove to be a valuable strategic therapy against P. aeruginosa infection. While many small molecules have been synthesized and tested as SYK inhibitors, it has been reported that some unwanted side effects are associated with its application along with a number of cautionary signs. The therapeutic action of some SYK inhibitors has already been demonstrated in clinical trials, which are currently in the advanced phase.
However, blocking the inflammatory pathway in CF might affect host defense mechanisms, which can be detrimental for CF patients. Moreover, it is unknown how SYK is regulated in CF cells, both epithelial and immune cells; this is a key question that needs to be addressed. Indeed, studies on breast cancer patients have reported a significant presence of different variations of the SYK gene which are associated with breast cancer pathogenesis [38]. The role of SYK in cellular responses to P. aeruginosa in infected animal models with a CFTR deficiency is completely unknown. Regarding host protection against P. aeruginosa, there are no published data assessing the effect of SYK inhibitors on the bactericidal activity of macrophages and neutrophils against P. aeruginosa. Further research is required to discover the capability of inhibition of SYK in animal models. This will demonstrate its effect on P. aeruginosa infection and the associated inflammatory responses which contribute significantly to the pathogenesis of P. aeruginosa pulmonary infections.
Disclosure Statement
The author does not have any conflict of interests to declare.
Acknowledgements
This work was supported by a scholarship award granted to A. Alhazmi, Jazan University, by the Saudi Arabian Cultural Bureau in Canada.
References
- 1.Taniguchi T, Kobayashi T, Kondo J, Takahashi K, Nakamura H, Suzuki J, et al. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991;266:15790–15796. [PubMed] [Google Scholar]
- 2.Kyttaris VC, Tsokos GC. Syk kinase as a treatment target for therapy in autoimmune diseases. Clin Immunol. 2007;124:235–237. doi: 10.1016/j.clim.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby C, Geahlen RL, Schreiber AD. Stimulation of macrophage Fc gamma RIIIA activates the receptor-associated protein tyrosine kinase Syk and induces phosphorylation of multiple proteins including p95Vav and p62/GAP-associated protein. J Immunol. 1994;152:5429–5437. [PubMed] [Google Scholar]
- 4.Benhamou M, Gutkind JS, Robbins KC, Siraganian RP. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci USA. 1990;87:5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchcroft JE, Geahlen RL, Deanin GG, Oliver JM. Fc epsilon RI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proc Natl Acad Sci USA. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kepley CL, Wilson BS, Oliver JM. Identification of the Fc epsilonRI-activated tyrosine kinases Lyn, Syk, and Zap-70 in human basophils. J Allergy Clin Immunol. 1998;102:304–315. doi: 10.1016/s0091-6749(98)70100-9. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan SR, Huang M, Berton G. Signaling by adhesion in human neutrophils: activation of the p72syk tyrosine kinase and formation of protein complexes containing p72syk and Src family kinases in neutrophils spreading over fibrinogen. J Immunol. 1997;158:1902–1910. [PubMed] [Google Scholar]
- 9.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan S, Warke VG, Nambiar MP, Tsokos GC, Farber DL. The FcR gamma subunit and Syk kinase replace the CD3 zeta-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J Immunol. 2003;170:4189–4195. doi: 10.4049/jimmunol.170.8.4189. [DOI] [PubMed] [Google Scholar]
- 11.Brumbaugh KM, Binstadt BA, Billadeau DD, Schoon RA, Dick CJ, Ten RM, Leibson PJ. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J Exp Med. 1997;186:1965–1974. doi: 10.1084/jem.186.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedlik C, Orbach D, Veron P, Schweighofer E, Colucci F, Gamberale R, et al. A critical role for Syk protein tyrosine kinase in Fc receptor-mediated antigen presentation and induction of dendritic cell maturation. J Immunol. 2003;170:846–852. doi: 10.4049/jimmunol.170.2.846. [DOI] [PubMed] [Google Scholar]
- 13.Watson SP, Gibbins J. Collagen receptor signalling in platelets: extending the role of the ITAM. Immunol Today. 1998;19:260–264. doi: 10.1016/s0167-5699(98)01267-5. [DOI] [PubMed] [Google Scholar]
- 14.Harrison ML, Isaacson CC, Burg DL, Geahlen RL, Low PS. Phosphorylation of human erythrocyte band 3 by endogenous p72syk. J Biol Chem. 1994;269:955–959. [PubMed] [Google Scholar]
- 15.Yamada T, Fujieda S, Yanagi S, Yamamura H, Inatome R, Sunaga H, Saito H. Protein-tyrosine kinase Syk expressed in human nasal fibroblasts and its effect on RANTES production. J Immunol. 2001;166:538–543. doi: 10.4049/jimmunol.166.1.538. [DOI] [PubMed] [Google Scholar]
- 16.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 17.Tsuge T, Suzuki Y, Shimokawa T, Horikoshi S, Okumura K, Ra C, Tomino Y. Monocyte chemoattractant protein (MCP)-1 production via functionally reconstituted Fc α receptor (CD89) on glomerular mesangial cells. Inflamm Res. 2003;52:428–432. doi: 10.1007/s00011-003-1200-x. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchida S, Yanagi S, Inatome R, Ding J, Hermann P, Tsujimura T, et al. Purification of a 72-kDa protein-tyrosine kinase from rat liver and its identification as Syk: involvement of Syk in signaling events of hepatocytes. J Biochem. 2000;127:321–327. doi: 10.1093/oxfordjournals.jbchem.a022610. [DOI] [PubMed] [Google Scholar]
- 19.Yanagi S, Inatome R, Ding J, Kitaguchi H, Tybulewicz VL, Yamamura H. Syk expression in endothelial cells and their morphologic defects in embryonic Syk-deficient mice. Blood. 2001;98:2869–2871. doi: 10.1182/blood.v98.9.2869. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimura T, Yanagi S, Inatome R, Takano T, Ishihara I, Mitsui N, et al. Syk protein-tyrosine kinase is involved in neuron-like differentiation of embryonal carcinoma P19 cells. FEBS Lett. 2001;489:129–133. doi: 10.1016/s0014-5793(01)02097-x. [DOI] [PubMed] [Google Scholar]
- 21.Fluck M, Zurcher G, Andres AC, Ziemiecki A. Molecular characterization of the murine syk protein tyrosine kinase cDNA, transcripts and protein. Biochem Biophys Res Commun. 1995;213:273–281. doi: 10.1006/bbrc.1995.2126. [DOI] [PubMed] [Google Scholar]
- 22.Ulanova M, Puttagunta L, Marcet-Palacios M, Duszyk M, Steinhoff U, Duta F, et al. Syk tyrosine kinase participates in β1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- 23.Yi YS, Son YJ, Ryou C, Sung GH, Kim JH, Cho JY. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:270302. doi: 10.1155/2014/270302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulanova M, Marcet-Palacios M, Munoz S, Asfaha S, Kim MK, Schreiber AD, Befus AD. Involvement of Syk kinase in TNF-induced nitric oxide production by airway epithelial cells. Biochem Biophys Res Commun. 2006;351:431–437. doi: 10.1016/j.bbrc.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 26.Furlong MT, Mahrenholz AM, Kim KH, Ashendel CL, Harrison ML, Geahlen RL. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta. 1997;1355:177–190. doi: 10.1016/s0167-4889(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 27.Ulanova M, Duta F, Puttagunta L, Schreiber AD, Befus AD. Spleen tyrosine kinase (Syk) as a novel target for allergic asthma and rhinitis. Expert Opin Ther Targets. 2005;9:901–921. doi: 10.1517/14728222.9.5.901. [DOI] [PubMed] [Google Scholar]
- 28.Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One. 2009;4:e7259. doi: 10.1371/journal.pone.0007259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aval PS, Werner J, Cerqueira A, Balfour-Boehm J, Ulanova M. Piceatannol modulates lung epithelial cellular responses to Pseudomonas aeruginosa. Inflamm Allergy Drug Targets. 2013;12:297–307. doi: 10.2174/18715281113129990011. [DOI] [PubMed] [Google Scholar]
- 31.Alhazmi A, Choi J, Ulanova M. Syk inhibitor R406 downregulates inflammation in an in vitro model of Pseudomonas aeruginosa infection. Can J Physiol Pharmacol. 2018;96:182–190. doi: 10.1139/cjpp-2017-0307. [DOI] [PubMed] [Google Scholar]
- 32.Osorio F, Reise Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–664. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werninghaus K, Babiak A, Gross O, Holscher C, Dietrich H, Agger EM, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shakeel S, Mahjabeen I, Kayani MA, Faryal R. Association of SYK genetic variations with breast cancer pathogenesis. Asian Pac J Cancer Prev. 2013;14:3309–3314. doi: 10.7314/apjcp.2013.14.5.3309. [DOI] [PubMed] [Google Scholar]
- 39.Dong SW, Zhang P, Zhong RR, Liu Q. Expression of putative tumor suppressor gene spleen tyrosine kinase in esophageal squamous cell carcinoma. Clin Lab. 2013;59:647–653. doi: 10.7754/clin.lab.2012.120414. [DOI] [PubMed] [Google Scholar]
- 40.Wong BR, Grossbard EB, Payan DG, Masuda ES. Targeting Syk as a treatment for allergic and autoimmune disorders. Expert Opin Investig Drugs. 2004;13:743–762. doi: 10.1517/13543784.13.7.743. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Lau C, Wiehler S, Pow A, Mazzulli T, Gutierrez C, Proud D, Chow CW. Syk is downstream of intercellular adhesion molecule-1 and mediates human rhinovirus activation of p38 MAPK in airway epithelial cells. J Immunol. 2006;177:6859–6870. doi: 10.4049/jimmunol.177.10.6859. [DOI] [PubMed] [Google Scholar]
- 42.Bajpai M, Chopra P, Dastidar SG, Ray A. Spleen tyrosine kinase: a novel target for therapeutic intervention of rheumatoid arthritis. Expert Opin Investig Drugs. 2008;17:641–659. doi: 10.1517/13543784.17.5.641. [DOI] [PubMed] [Google Scholar]
- 43.Blease K. Targeting kinases in asthma. Expert Opin Investig Drugs. 2005;14:1213–1220. doi: 10.1517/13543784.14.10.1213. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda T, Yamamoto T, Kishi H, Yoshimura A, Muraguchi A. SOCS-1 can suppress CD3zeta- and Syk-mediated NF-AT activation in a non-lymphoid cell line. FEBS Lett. 2000;472:235–240. doi: 10.1016/s0014-5793(00)01444-7. [DOI] [PubMed] [Google Scholar]
- 45.Leseux L, Hamdi SM, Al Saati T, Capilla F, Recher C, Laurent G, Bezombes C. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- 46.Goodman PA, Burkhardt N, Juran B, Tibbles HE, Uckun FM. Hypermethylation of the spleen tyrosine kinase promoter in T-lineage acute lymphoblastic leukemia. Oncogene. 2003;22:2504–2514. doi: 10.1038/sj.onc.1206313. [DOI] [PubMed] [Google Scholar]
- 47.Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Ding YB, Chen GY, Xia JG, Wu ZY. Hypermethylation of Syk gene in promoter region associated with oncogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2004;10:1815–1818. doi: 10.3748/wjg.v10.i12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113:3154–3160. doi: 10.1182/blood-2008-07-166439. [DOI] [PubMed] [Google Scholar]
- 50.Oda A, Ochs HD, Lasky LA, Spencer S, Ozaki K, Fujihara M, Handa M, Ikebuchi K, Ikeda H. CrkL is an adapter for Wiskott-Aldrich syndrome protein and Syk. Blood. 2001;97:2633–2639. doi: 10.1182/blood.v97.9.2633. [DOI] [PubMed] [Google Scholar]
- 51.Krishnan S, Juang YT, Chowdhury B, Magilavy A, Fisher CU, Nguyen H, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181:8145–8152. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie HZ, Li LL, Ren JX, Zou J, Yang L, Wei YQ, Yang SY. Pharmacophore modeling study based on known spleen tyrosine kinase inhibitors together with virtual screening for identifying novel inhibitors. Bioorg Med Chem Lett. 2009;19:1944–1949. doi: 10.1016/j.bmcl.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 53.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 54.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 55.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 56.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 57.Rajan S, Saiman L. Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect. 2002;17:47–56. doi: 10.1053/srin.2002.31690. [DOI] [PubMed] [Google Scholar]
- 58.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 59.Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mall M, Hipper A, Greger R, Kunzelmann K. Wild type but not δF508 CFTR inhibits Na+ conductance when coexpressed in Xenopus oocytes. FEBS Lett. 1996;381:47–52. doi: 10.1016/0014-5793(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 61.Pier GB. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. Proc Natl Acad Sci USA. 2000;97:8822–8828. doi: 10.1073/pnas.97.16.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang A, Sharma A, Jen R, Hirschfeld AF, Chilvers MA, Lavoie PM, Turvey SE. Inflammasome-mediated IL-1β production in humans with cystic fibrosis. PLoS One. 2012;7:e37689. doi: 10.1371/journal.pone.0037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison F. Microbial ecology of the cystic fibrosis lung. Microbiology. 2007;153:917–923. doi: 10.1099/mic.0.2006/004077-0. [DOI] [PubMed] [Google Scholar]
- 64.Hauser AR. Pseudomonas aeruginosa: so many virulence factors, so little time. Crit Care Med. 2011;39:2193–2194. doi: 10.1097/CCM.0b013e318221742d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Luz S, Kongsuphol P, Mendes AI, Romeiras F, Sousa M, Schreiber R, et al. Contribution of casein kinase 2 and spleen tyrosine kinase to CFTR trafficking and protein kinase A-induced activity. Mol Cell Biol. 2011;31:4392–4404. doi: 10.1128/MCB.05517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendes AI, Matos P, Moniz S, Luz S, Amaral MD, Farinha CM, Jordan P. Antagonistic regulation of cystic fibrosis transmembrane conductance regulator cell surface expression by protein kinases WNK4 and spleen tyrosine kinase. Mol Cell Biol. 2011;31:4076–4086. doi: 10.1128/MCB.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stephens DJ, Banting G. Insulin dependent tyrosine phosphorylation of the tyrosine internalisation motif of TGN38 creates a specific SH2 domain binding site. FEBS Lett. 1997;416:27–29. doi: 10.1016/s0014-5793(97)01165-4. [DOI] [PubMed] [Google Scholar]
- 69.Gasparrini F, Molfetta R, Quatrini L, Frati L, Santoni A, Paolini R. Syk-dependent regulation of Hrs phosphorylation and ubiquitination upon FcεRI engagement: impact on Hrs membrane/cytosol localization. Eur J Immunol. 2012;42:2744–2753. doi: 10.1002/eji.201142278. [DOI] [PubMed] [Google Scholar]
- 70.Greenberg S. Modular components of phagocytosis. J Leukoc Biol. 1999;66:712–717. doi: 10.1002/jlb.66.5.712. [DOI] [PubMed] [Google Scholar]
- 71.Moniz S, Sousa M, Moraes BJ, Mendes AI, Palma M, Barreto C, et al. HGF stimulation of Rac1 signaling enhances pharmacological correction of the most prevalent cystic fibrosis mutant F508del-CFTR. ACS Chem Biol. 2013;8:432–442. doi: 10.1021/cb300484r. [DOI] [PubMed] [Google Scholar]
- 72.Farinha CM, Swiatecka-Urban A, Brautigan DL, Jordan P. Regulatory crosstalk by protein kinases on CFTR trafficking and activity. Front Chem. 2016;4:1. doi: 10.3389/fchem.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Highsmith WE, Burch LH, Zhou Z, Olsen JC, Boat TE, Spock A, et al. A novel mutation in the cystic fibrosis gene in patients with pulmonary disease but normal sweat chloride concentrations. N Engl J Med. 1994;331:974–980. doi: 10.1056/NEJM199410133311503. [DOI] [PubMed] [Google Scholar]
- 74.Highsmith WE, Jr, Burch LH, Zhou Z, Olsen JC, Strong TV, Smith T, et al. Identification of a splice site mutation (2789 +5 G > A) associated with small amounts of normal CFTR mRNA and mild cystic fibrosis. Hum Mutat. 1997;9:332–338. doi: 10.1002/(SICI)1098-1004(1997)9:4<332::AID-HUMU5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 75.Tzetis M, Efthymiadou A, Doudounakis S, Kanavakis E. Qualitative and quantitative analysis of mRNA associated with four putative splicing mutations (621 + 3A–>G, 2751 + 2T–>A, 296 + 1G–>C, 1717–9T–>C-D565G) and one nonsense mutation (E822X) in the CFTR gene. Hum Genet. 2001;109:592–601. doi: 10.1007/s00439-001-0631-0. [DOI] [PubMed] [Google Scholar]
- 76.Ramalho AS, Beck S, Meyer M, Penque D, Cutting GR, Amaral MD. Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis. Am J Respir Cell Mol Biol. 2002;27:619–627. doi: 10.1165/rcmb.2001-0004OC. [DOI] [PubMed] [Google Scholar]
- 77.Nixon LS, Yung B, Bell SC, Elborn JS, Shale DJ. Circulating immunoreactive interleukin-6 in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1764–1769. doi: 10.1164/ajrccm.157.6.9704086. [DOI] [PubMed] [Google Scholar]
- 78.Hawdon NA, Aval PS, Barnes RJ, Gravelle SK, Rosengren J, Khan S, Ciofu O, Johansen HK, Hoiby N, Ulanova M. Cellular responses of A549 alveolar epithelial cells to serially collected Pseudomonas aeruginosa from cystic fibrosis patients at different stages of pulmonary infection. FEMS Immunol Med Microbiol. 2010;59:207–220. doi: 10.1111/j.1574-695X.2010.00693.x. [DOI] [PubMed] [Google Scholar]
- 79.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li JD, Feng W, Gallup M, Kim JH, Gum J, Kim Y, Basbaum C. Activation of NF-κB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans DJ, Frank DW, Finck-Barbancon V, Wu C, Fleiszig SM. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect Immun. 1998;66:1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- 84.Esen M, Grassme H, Riethmuller J, Riehle A, Fassbender K, Gulbins E. Invasion of human epithelial cells by Pseudomonas aeruginosa involves src-like tyrosine kinases p60Src and p59Fyn. Infect Immun. 2001;69:281–287. doi: 10.1128/IAI.69.1.281-287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saiman L, Mehar F, Niu WW, Neu HC, Shaw KJ, Miller G, Prince A. Antibiotic susceptibility of multiple resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin Infect Dis. 1996;23:532–537. doi: 10.1093/clinids/23.3.532. [DOI] [PubMed] [Google Scholar]
- 86.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 87.Lin YC, Huang DY, Wang JS, Lin YL, Hsieh SL, Huang KC, Lin WW. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol. 2015;97:825–835. doi: 10.1189/jlb.3HI0814-371RR. [DOI] [PubMed] [Google Scholar]
- 88.Dinarello CA. Interleukin-1β and the autoinflammatory diseases. N Engl J Med. 2009;360:2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- 89.Alhazmi A. NOD-like receptor(s) and host immune responses with Pseudomonas aeruginosa infection. Inflamm Res. 2018;67:479–493. doi: 10.1007/s00011-018-1132-0. [DOI] [PubMed] [Google Scholar]
- 90.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaumouille V, Farkash Y, Jaqaman K, Das R, Lowell CA, Grinstein S. Actin cytoskeleton reorganization by Syk regulates Fcgamma receptor responsiveness by increasing its lateral mobility and clustering. Dev Cell. 2014;29:534–546. doi: 10.1016/j.devcel.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Ziffle JA, Lowell CA. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood. 2009;114:4871–4882. doi: 10.1182/blood-2009-05-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. 2016;8:550–563. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konstan MW, Doring G, Heltshe SL, Lands LC, Hilliard KA, Koker P, et al. Investigators and Coordinators of BI Trial 543.45 A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros. 2014;13:148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doring G, Bragonzi A, Paroni M, Akturk FF, Cigana C, Schmidt A, et al. BIIL 284 reduces neutrophil numbers but increases P.aeruginosa bacteremia and inflammation in mouse lungs. J Cyst Fibros. 2014;13:156–163. doi: 10.1016/j.jcf.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baldwin AG, Brough D, Freeman S. Inhibiting the inflammasome: a chemical perspective. J Med Chem. 2016;59:1691–1710. doi: 10.1021/acs.jmedchem.5b01091. [DOI] [PubMed] [Google Scholar]
- 101.Deng GM, Kyttaris VC, Tsokos GC. Targeting Syk in autoimmune rheumatic diseases. Front Immunol. 2016;7:78. doi: 10.3389/fimmu.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng GM, Tsokos GC. The role of Syk in cutaneous lupus erythematosus. Exp Dermatol. 2016;25:674–675. doi: 10.1111/exd.13018. [DOI] [PubMed] [Google Scholar]
- 103.Ma TK, McAdoo SP, Tam FW. Spleen tyrosine kinase: a crucial player and potential therapeutic target in renal disease. Nephron. 2016;133:261–269. doi: 10.1159/000446879. [DOI] [PubMed] [Google Scholar]
- 104.Currie KS, Kropf JE, Lee T, Blomgren P, Xu J, Zhao Z, et al. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem. 2014;57:3856–3873. doi: 10.1021/jm500228a. [DOI] [PubMed] [Google Scholar]
- 105.Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28:331–346. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 106.Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151:939–941. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]