Abstract
The growth and metastasis of tumors is dependent on angiogenesis. C-type lectins are carbohydrate-binding proteins with a diverse range of functions. The C-type lectin family XIV members are transmembrane glycoproteins, and all four members of this family have been reported to regulate angiogenesis, although the detailed mechanism of action has yet to be completely elucidated. They interact with extracellular matrix proteins and mediate cell-cell adhesion by their lectin-like domain. The aim of the present study was to summarize the available information on the function and mechanism of C-type lectin family XIV in angiogenesis and discuss their potential as targets for cancer therapy.
Keywords: C-type lectin family XIV, angiogenesis, CLEC14A, thrombomodulin, CD93, endosialin
1. Introduction
Vascular development begins with the differentiation of mesodermal cells into endothelial cell precursors (angioblasts), which form primary vessels de novo by a process known as vasculogenesis (1). The primary vessels are subsequently remodeled by the sprouting and branching of new blood vessels, a process known as angiogenesis. Physiologically, angiogenesis establishes the first vascular tree and adequate vasculature for the growth and development of organs in the embryo (2), whilst in adults, angiogenesis occurs during the ovarian cycle and wound healing (3). The process is controlled by balancing inducers and inhibitors of angiogenesis (4). Cancer cells use angiogenesis to fulfil the increased need for nutrients and oxygen to the growing tumor. Angiogenesis also promotes tumor invasion and metastasis, and has been described as one of the six hallmarks of cancer (5).
Angiogenesis begins with the activation of quiescent endothelial cells (ECs) in response to angiogenic stimuli. A number of proteins are important for angiogenesis. The VEGF family of secreted proteins and their receptors, Fibroblast growth factors and Notch signaling are some of the most well studied regulators of angiogenesis. Platelet derived growth factor, the angiopoietins and tie receptors are associated with vessel maturation (6). The extracellular matrix (ECM) serves a pivotal role in the regulation of both physiological and pathological angiogenesis (7). Endothelial cell-cell adhesion and adhesion with the ECM are essential to establish the appropriate cellular configuration for growth, survival and differentiation. Cell adhesion molecules, including integrins, cadherin, immunoglobulin families and selectin are critically involved in angiogenesis (8). In absence of appropriate cell contact, the ECs may undergo programmed cell death or unable to form new capillary blood vessels and extension and maturation of new vessels (8,9). Activated ECs secrete proteinases to breakdown the surrounding basement matrix and invade the ECM (1,10). Once free from the ECM, ECs proliferate and migrate towards chemotactic and angiogenic stimuli. The newly formed vessels are stabilized by basement membrane synthesis and the recruitment of pericytes, and fresh sprouts fuse to establish blood flow (11–13).
The C-type lectin family XIV members are expressed on angiogenic blood vessels and are vital for cell-cell adhesion and cell-ECM interactions during angiogenesis. The ECM also releases proteiolytic enzymes that results in degradation of matrix molecules and soluble factors that promote angiogenesis (7). The C-type lectin family XIV members are associated with increased expression of ECM degrading enzymes like MMPs and plasminogen activators. They have been associated with increased rate of angiogenesis in a variety of cancers. These proteins are also implicated in other diseases involving endothelial dysfunction and have been used as a biomarker in these diseases. High plasma levels of thrombomodulin has been observed in preeclampsia, diffuse intravascular coagulation, Shiga toxin-producing E. coli (STEC)-induced and atypical hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, scleroderma-associated pulmonary hypertension, and arterial hypertension (14–19). The role of endosialin in rheumatoid arthritis and Salmonella infection is well established (20,21). CD93 expression is found to be altered in systemic lupus erythematosus, rheumatoid arthritis and coronary artery disease (22–24). In this review study, however, we have focused primarily on the role of these proteins in regulating tumour angiogenesis.
2. C-type lectin family XIV
C-type lectins are calcium (Ca2+)-dependent carbohydrate binding proteins whose activity is mediated by a carbohydrate recognition domain (CRD), a compact module with a globular structure (25). It was later observed that not all CRD-containing proteins require Ca2+, or bind carbohydrates, and such proteins are said to possess C-type lectin-like domains (CTLDs) (26). The CTLD-containing proteins have been classified into 17 groups based on their CTLD architecture and the evolutionary and functional associations (27–29). The angiogenic roles of C-type lectin family XIV members are not clearly understood. The family comprises of four members [C-type lectin domain family 14 member A (CLEC14A), thrombomodulin, cluster of differentiation 93 (CD93) and endosialin] (30) (Fig. 1), which are cell surface glycoproteins with a single CTLD and a variable number of epidermal growth factor (EGF)-like repeats. CLEC14A, thrombomodulin and CD93 are expressed by ECs, whereas endosialin is expressed by endothelial proximal stromal cells.
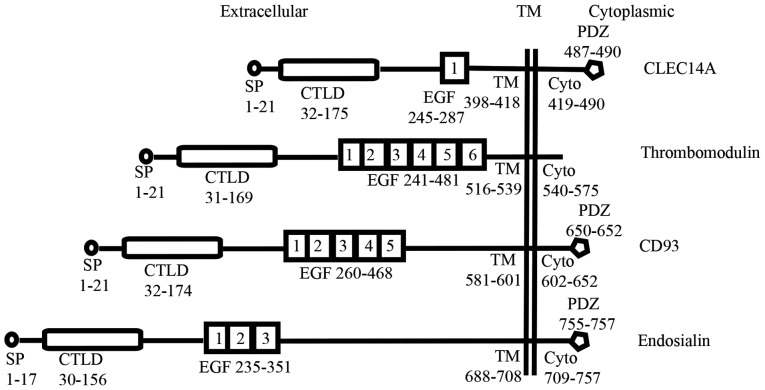
Figure 1.
C-type lectin family XIV group. All the four members have a set of conserved domains such as the CTLD and different number of EGF like repeat domains. Some members also have a PDZ binding motif in the C-terminal cytoplasmic tail. Location of the domains in the full-length proteins with amino acid residues is given in numbers. SP, signal peptide; CTLD, C-type lectin-like domain; EGF, Epidermal growth factor-like domain; TM, Transmembrane domain; Cyto, Cytoplasmic domain; PDZ, PDZ binding motif.
3. CLEC14A
CLEC14A is a transmembrane glycoprotein containing an extracellular signal peptide, a CTLD, a sushi-like domain, a single EGF-like domain, a mucin-like domain, a single transmembrane domain and an intracellular cytoplasmic domain. Human CLEC14a is an intronless gene located on chromosome 14q21.1, and CLEC14A is expressed specifically in the embryonic vasculature of mice and zebrafish, and by human ECs (31). Reverse transcription-quantitative PCR analysis revealed that CLEC14A expression begins from 12 h post-fertilization in zebrafish embryos, which coincides with the generation of hemangioblasts. Also, CLEC14A expression level was shown to increase in the later stages of angiogenic development (32). CRISPR-Cas9-mediated knockout of clec14a in zebrafish resulted in the malformation of inter-segmental vessels (ISVs), and the knockout of both cd93 and clec14a resulted in the inhibition of cadherin 5 expression in ISVs (33). Furthermore, the CLEC14A expression level was significantly higher in the tumor vasculature compared with the normal vasculature (32). CLEC14A is one of the primary genes of the tumor angiogenesis signature, highly expressed in head and neck squamous cell carcinoma, breast cancers and clear cell renal cell carcinoma (34), and is therefore considered to be a tumor endothelial marker (TEM). Its expression was also shown to increase in response to hypoxia in HUVECs (35).
CLEC14A has also been reported to regulate pro-angiogenic phenotypes such as filopodia formation, cell migration and tube formation in HUVECs (31,32); it localizes to the inter-cellular boundary and regulates cell adhesion through its CTLD (31). Targeted neutralization of CLEC14A using an anti-CTLD antibody was shown to inhibit endothelial cell migration, cell-cell contact and tube formation by blocking CTLD-CTLD interactions, and downregulating CLEC14A expression at the endothelial cell surface (36,37). A Stable Isotope Labelling with Amino Acids in Cell Culture-based proteomics study showed the upregulation of CLEC14A expression during tubule morphogenesis, and the analysis of post-translational modifications of CLEC14A identified a phosphorylation site at Ser483, near the PDZ-binding domain in the cytoplasmic tail (38). The PDZ domain serves a vital role in protein-protein interactions (39), although no functional studies have been reported for the phosphorylation site and the PDZ domain of CLEC14A.
Secretory CLEC14A co-localizes with fibronectin (FN1), laminin alpha 4 (LAMA4) and multimerin 2 (MMRN2) in the ECM, and its expression is upregulated during tumor angiogenesis in spontaneous mouse tumors (38). FN1 and LAMA4 were also reportedly deregulated during tumor angiogenesis (40,41), and MMRN2 was shown to suppress vascular endothelial growth factor A (VEGFA)/Vascular endothelial growth factor receptor 2 (VEGFR2) signaling by sequestering VEGFA (42). However, the pro-angiogenic properties of MMRN2 have also been reported (38,43,44). Furthermore, antibody-mediated disruption of the interaction between CLEC14A and MMRN2 inhibited sprouting angiogenesis and tumor growth (45). As an ECM protein, MMRN2 binds to the CTLD of CLEC14A; CLEC14A-CTLD is also reported to interact with Hsp70-1A and to be crucial for Hsp70-1A-induced angiogenesis via extracellular-signal-regulated kinase (ERK) activation (46). Moreover, CLEC14A is cleaved by rhomboid-like protein 2 (a membrane-embedded proteolytic enzyme), and the cleaved extracellular domain has sprout-inhibiting and anti-migratory properties. This inhibition is thought to have been due to competition between the cleaved extracellular domain and wild type forms of CLEC14A for the binding of MMRN2 (47).
In contrast to the above observations, increased angiogenesis and lymphangiogenesis were observed in CLEC14A-knockout mice, and CLEC14A-deficient mice exhibited abnormal tumor vasculature and reduced survival of tumor-bearing mice. Additionally, the loss of CLEC14A was attributed to the loss of VEGFR3 expression and suppressed Notch/Dll4 and Notch target gene expression (48). Furthermore, the deletion of EC-specific VEGFR3 was shown to induce hypervascularity, and VEGFR-3 knockdown using siRNA, followed by VEGF treatment, increased the level of VEGFR-2 phosphorylation in HUVECs (49). The role of CLEC14A may be context-dependent, and future studies to identify CLEC14A-interacting partners (of both to the extracellular domain and the cytoplasmic domain) and their signaling pathways may enhance understanding of their precise roles in angiogenesis.
4. Thrombomodulin
Thrombomodulin (also known as CD141) is a membrane-bound glycoprotein with an N-terminal signal peptide, a CTLD, six tandem EGF-like domain repeats, an O-glycosylation site-rich domain, a transmembrane domain and a short cytoplasmic C-terminal loop (50,51). Thrombomodulin is an intronless gene (location, chromosome 20p11.21) initially identified as a gene expressed in the vascular endothelium (52). It was later discovered to also be expressed in smooth muscle cell lines (53), and by both circulating and tissue mononuclear phagocytes (54). Additionally, treatment with VEGF also increased the expression level of thrombomodulin in human aortic ECs (55). The CTLD of thrombomodulin was reported to mediate Ca2+-dependent cell-cell adhesion, and antibody-targeted inhibition of the CTLD prevented cell-cell contact, whereas an antibody towards the EGF domain of thrombomodulin did not (56). In addition, thrombomodulin was shown to co-localize with actin filaments, and cell-cell adhesion was abolished by mannose, chondroitin sulfate A and chondroitin sulfate C administration (56).
It was postulated that the lectin-like domain of thrombomodulin (expressed at the tumor surface) interacted with cell membrane and ECM proteins, and also facilitated cell-cell adhesion (57,58). The CTLD of thrombomodulin also interacts with the ECM protein fibronectin in the tumor vasculature, enhancing cell adhesion and migration. It has also been reported to promote the phosphorylation of focal adhesion kinase 1 (FAK) and the expression of matrix metalloproteinase 9 (MMP9) (59). The role of FAK in angiogenesis has also been reported (60,61).
Knockdown of thrombomodulin in HaCaT cells inhibited E-cadherin trafficking to the cell membrane, bestowing a more fibroblast-like phenotype (62). In another study, the recombinant lectin-like domain of TM-TM domain 1 (rTMD1) was found to inhibit HUVEC tube formation by Matrigel assays, as well as disrupting the interaction between rTMD1 and Lewis Y Ag (LeY)-modified EGFR, resulting in the inhibition of EGF-mediated EGFR signaling and angiogenesis. This was believed to be due to rTMD1-associated interference of LeY (a cell surface tetra-saccharide) in endothelial cell connection and capillary formation (63).
A soluble form of thrombomodulin containing the CTLD has been shown to retard tumor cell invasiveness, whereas soluble thrombomodulin lacking this domain was unable to inhibit cell invasion, suggesting an anti-metastatic role for the CTLD of thrombomodulin (64). The CTLD of thrombomodulin was also shown to suppress lipopolysaccharide-induced ERK1/2 phosphorylation (65). Additionally, thrombomodulin expression was inversely correlated with tumor cell proliferation in lung squamous cell carcinoma (66), esophageal squamous cell carcinoma (67), hepatocellular carcinoma (57), colorectal cancer (68), and malignant melanoma (69); its expression level was also increased in a number of other cancer types, including colorectal cancer, pancreatic cancer, mammary carcinoma, leukemia (70) and glioblastoma (71).
The CTLD and the cytoplasmic domain of thrombomodulin were also discovered to be necessary for reduced cell proliferation (69); it was revealed that the cytoplasmic domain of thrombomodulin interacted with the N-terminal membrane-cytoskeleton linker ezrin/radixin/moesin (ERM) family protein ezrin. Thrombomodulin, ezrin and F-actin were shown to co-localize at intercellular filopodia, and the interaction between ezrin and CD44 has been reported to facilitate cancer cell migration. It was also hypothesized that the thrombomodulin-ezrin interaction may prevent the binding of CD44 to ezrin, resulting in the reduced migration of thrombomodulin-expressing cells compared with thrombomodulin knockdown cells (72).
In other epithelial and tumor cell lines, the expression of thrombomodulin and Snail was inversely correlated; Snail is a transcription factor involved in epithelial mesenchymal transition (EMT) that has been shown to bind the thrombomodulin promoter and suppress its expression (62). Thrombomodulin was also able to reverse EMT by upregulating E-cadherin and downregulating N-cadherin expression levels in lung cancer cells (73). By contrast, the recombinant thrombomodulin fragment TMD23 (with a 6-tandem EGF-like domain and O-glycosylation site-rich domain) was reported to stimulate angiogenesis (63,74). The C loop of the C-terminal sub-domain of the fifth EGF-like domain of TMD23 has pro-angiogenic and cytoprotective effects, in a G protein-coupled receptor 15-dependent manner (75). Angiogenesis was also mediated by the phosphorylation of ERK1/2, p38, protein kinase B (Akt) and Endothelial nitric oxide synthase (eNOS) (74), and by Fibroblast growth factor receptor 1-A (76). rTMD23 was reported to stimulate the endothelial cell expression of MMPs and plasminogen activators that mediate ECM degradation, and subsequently, angiogenesis (74). This suggests that rTMD23 is responsible for cellular proliferation and migration, and that the CTLD may possess anti-angiogenic properties.
5. CD93
CD93 (also known as complement component C1q receptor and AA4.1) is a type I transmembrane glycoprotein with one C-type lectin-like domain, five tandem EGF-like domain repeats, a serine threonine-rich mucin-like domain, a transmembrane domain and a cytoplasmic domain (77). CD93 is located on chromosome 20p11.21 and has two exons separated by a single intron (78). CD93 was identified as one of the top 20 genes in the core human primary tumor angiogenesis signature (34). Its expression is prominent in ECs and certain hematopoietic subsets, including myeloid cells, platelets and hematopoietic stem cells (79–81). It is also highly expressed in tumor ECs, but exhibits low expression levels in non-proliferating ECs (34,82–85). A soluble form of the CD93 ectodomain (containing the CTLD and EGF-like domain repeats) was detected in normal human plasma (80) and during inflammatory stimulation in vivo (86). In situ hybridization of mouse CD93 revealed its expression in the vascular endothelium of 9 day old embryos; this correlated with the remodeling of blood vessels in the intersomitic branches of the dorsal aorta and developing perineural plexus, suggesting an angiogenic role for CD93 (87).
The CTLD of CD93 is essential for intercellular adhesion not sensitive to calcium chelators (79). Also, CD93 knockdown inhibited tube formation, migration and adhesion of ECs (82), and the growth of orthotopically-implanted syngeneic GL261 gliomas was retarded in CD93−/− mice, which was associated with abnormal tumor vessel growth (82).
The cytoplasmic domain of CD93 interacts with the ERM protein moesin, establishing a link between CD93 and actin that contributes to cytoskeletal reorganization, an essential process for cellular adhesion (88). CD93 also interacts with dystroglycan, an ECM receptor and laminin-binding protein. Through dystroglycan, the tyrosine residues in the cytoplasmic domain of CD93 are phosphorylated following adhesion to laminin, and this phosphorylation is necessary for endothelial cell migration (89). In addition to the CTLD, the DX domain (a 79 residue-long stretch situated between the CTLD and 5 EGF-like domain repeats) is necessary for the interaction between CD93 and MMRN2; blocking this interaction by targeting the DX domain retarded the angiogenesis (90). In endothelial filopodia, interaction with MMRN2 stabilizes CD93 to prevent the shedding of its extracellular domain. This stable complex is required for the activation of β1 integrin, which initiates the phosphorylation of FAK and the organization of fibronectin into fibrillar structures (43).
The recombinant CD93 protein rCD93-D23 (containing the EGF domain and a serine-threonine rich-mucin-like domain) induced HUVEC proliferation and migration via the ERK1/2, PI3K/Akt/eNOS pathways and EGFR signaling; the CTLD of this protein did not influence angiogenesis in vivo (or did so to a moderate degree only), as indicted by the removal of the entire ectodomain (including the CTLD), and suggesting that the CTLD of CD93 may possess an anti-angiogenic function (91). Monoclonal antibody-targeting of the extracellular domain of CD93 inhibited the proliferation, migration and sprouting of ECs without influencing endothelial cell survival and the inhibition of angiogenesis was suggested to result from the prohibition of cell adhesion. The epitope recognized by this antibody is in the region overlapping the CTLD and DX domain, and lies outside of the EGF domain; it does not impair CD93-dependent EGFR activation (which is dependent on the EGF domain) suggesting that CD93 may possess a different angiogenic function as a membrane-intercalated protein than insoluble form (92).
6. Endosialin
The extracellular domain of endosialin [also known as tumor endothelial marker 1 (TEM1) and CD248] comprises a CTLD, a Sushi domain (also known as a short consensus repeat or complement control protein domain) and three EGF-like domains, followed by a transmembrane and a cytoplasmic domain (93). Endosialin was initially identified as a cell surface glycoprotein and TEM (94), though further studies have suggested endosialin as a marker of cancer-associated fibroblasts (CAFs) and tumor vessel associated mural cells, rather than a mesenchymal stem cell (MSC) marker. The expression of endosialin by both CAFs and MSCs indicates the involvement of the latter in tumor stroma formation via differentiation into tumor stromal fibroblasts (95). Furthermore, endosialin-downregulated fibroblasts showed a platelet-derived growth factor-BB-mediated reduction in migration and proliferation (96).
The reported binding partners of endosialin are metastasis-related protein Mac2-BP/90K (97) and MMRN2 (44). Its CTLD interacts with ECM proteins such as collagen type I/IV and fibronectin, both of which mediate cell adhesion and migration. CHO cells overexpressing endosialin proliferated in clusters to form web-like structures, that formed larger clusters over time compared with normal CHO cells, which proliferate as singular cells (98). Endosialin-null mice showed normal physiological angiogenesis; however, there was a reduction in tumor growth, an increased number of small and immature tumor vessels and a decreased number of larger and mature vessels, highlighting the importance of endosialin in tumor microvasculature maturation (99). Moreover, mice lacking the cytoplasmic domain of endosialin exhibited reduced expression levels of VEGF, hypoxia-inducible factor-1α (HIF1α), placental growth factor (PlGF), MMP9 (20), and increased expression levels of the tumor suppressor transgelin (SM22α) and the downstream effector of Notch (100). VEGF, HIF1α, P1GF and MMP9 are pro-angiogenic, whereas SM22α is known to have tumor suppressive properties (101). Depending on the tissue and cellular context, Notch can be either oncogenic or tumor suppressive (102). It was also revealed that endosialin expression was induced in hypoxic conditions and regulated by HIF2α; endosialin transcription was also enhanced by the interaction of HIF2α with the proto-oncoprotein protein C-ets-1 (103).
7. Conclusions and prospects
As a number of pathways are able to compensate for the VEGF-targeted inhibition of angiogenesis, anti-angiogenic therapies that target VEGF alone are not sufficient (104). Acquired resistance to the inhibition of VEGF signaling, and its toxicity towards normal physiology demand a broader range of therapeutic approaches, targeting multiple aspects of angiogenesis.
The members of the C-type lectin family XIV are transmembrane proteins expressed at the cell surface, and are therefore relatively easy to target. With the exception of thrombomodulin, C-type lectin family XIV members are predominantly expressed by tumor ECs. Endosialin is expressed by the tumor vasculature, tumor stromal cells and MSCs, making it an attractive target for anti-angiogenic therapy in various types of tumor (95). CLEC14A induces filopodia and tube formation (32), and its interaction with MMRN2 promotes tumor growth and angiogenesis (45). Thrombomodulin interacts with fibronectin during tumor angiogenesis and maintains the endothelial tube structure (59). CD93 interacts with MMRN2, fibronectin fibrils and α5β1 integrin, promoting angiogenesis via FAK phosphorylation (43). The interaction between the cytoplasmic tail of CD93 and moesin induces cytoskeletal reorganization (88), and the interaction between endosialin, collagen and fibronectin mediates cell adhesion and migration (98).
As discussed, all members of the C-type lectin family XIV interact with ECM proteins and support endothelial cell migration. Inhibiting the function of these proteins may lead to reduced endothelial cell migration and angiogenesis. Particular family members also promote downstream signaling mechanisms, such as the phosphorylation of FAK and an increase in the expression levels of MMP9 and plasminogen activators (Fig. 2). The C-type lectin family XIV members have been reported to enhance angiogenesis in different cancer types (Table I); however, these findings do not clearly illustrate the mechanisms by which they regulate angiogenesis. The CTLD has been shown to exert both pro-and anti-angiogenic activity in different members of C-type lectin family XIV. Additionally, the EGF-like domain is necessary for their angiogenic capacity, and may be a potential target for anti-angiogenic therapy.
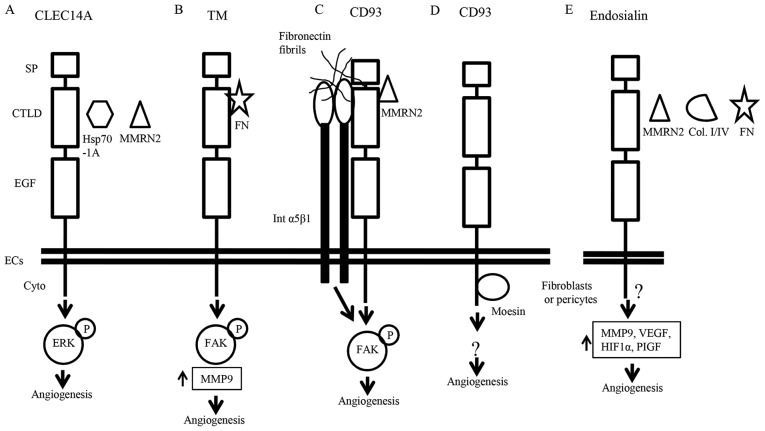
Figure 2.
Mechanism of regulation of angiogenesis by the members of C-type lectin family XIV. (A) Interaction of CLEC14A with Hsp70-1A leads to ERK phosphorylation and angiogenesis. CLEC14A interaction with MMRN2 promotes angiogenesis by unknown mechanisms. (B) Interaction between TM and fibronectin promotes angiogenesis by FAK phosphorylation and increased MMP9 production. (C) CD93 interaction with MMRN2, fibronectin fibril and α5β1 integrin promotes angiogenesis by FAK phosphorylation. (D) The interaction of the cytoplasmic domain of CD93 with moesin leads to cytoskeletal reorganization. (E) Endosialin interaction with MMRN2, collagen I/IV and fibronectin promotes angiogenesis by unknown mechanisms. Endosialin may have a role in expression of MMP9 and PlGF. TM, thrombomodulin; FN, fibronectin; Col. I/IV, collagen I/IV; Cyto, cytoplasmic domain.
Table I.
Function of C-type lectin family XIV members in different cancers.
| C-type lectin family XIV | Cell type | Effect |
|---|---|---|
| CLEC14A | Squamous cell carcinoma (34,38), cervical cancer (38), pancreatic neuroendocrine tumors (38), clear cell renal cell carcinomas (34), breast cancer (34) | Increased angiogenesis and metastasis |
| Thrombomodulin | Lung squamous cell carcinoma (66), oesophageal squamous cell carcinoma (67), hepatocellular carcinoma (57), colorectal cancer (68), malignant melanoma (69) | Decreased tumor cell proliferation and invasion |
| Leukaemia (70), pancreatic cancer (70), colorectal cancer (70), mammary carcinoma (70), glioblastoma (71) | Increased invasion and angiogenesis | |
| CD93 | Glioblastoma (43), nasopharyngeal carcinoma (83) | Increased angiogenesis and tumor growth |
| Endosialin | T241 fibrosarcomas (100), Lewis lung Carcinomas (100), cervical cancer cell line HeLa (94), amelanotic melanoma cell line A375 (94), neuroblastoma cell line LA1-5s (94) | Increased tumor growth and angiogenesis, tumor microvasculature maturation |
Improved characterization of the structural motifs and domains of members of C-type lectin family XIV will aid in the understanding of their mechanisms of signal transduction and angiogenesis. Specific binding partners of the family are known (Table II), yet detailed mechanisms of the roles of these proteins in angiogenesis require further elucidation. It is evident that members of C-type lectin family XIV are important regulators of physiological and pathological angiogenesis, and therefore present as attractive therapeutic targets.
Table II.
Co-localization/binding partners of C-type lectin family XIV members.
| Protein | Intracellular | Extracellular |
|---|---|---|
| CLEC14A | Not reported | Fibronectin (38), Laminin alpha 4 (38), MMRN2 (38,44,45), Hsp70-1A (46) |
| Thrombomodulin | Actin (56), Ezrin (72) | Fibronectin (59) |
| CD93 | Moesin (88) | Dystroglycan (89), MMRN2 (43,44,90), β1 integrin (43), Fibronectin (43) |
| Endosialin | Not reported | MMRN2 (44), Mac2-BP/90K (97), Collagen type I, IV (98), Fibronectin (98) |
Acknowledgements
The authors would like to thank Mr S. Rajivgandhi (Institute of Life Sciences, Bhubaneswar) for comments on the manuscript.
Funding
RKS is supported by DBT grant (6242-P64/RGCB/PMD/DBT/RJKS/2015), SERB-EMR (EMR/2016/003780) and intramural funds from ILS, which is an autonomous institute of DBT, Government of India. SB is a recipient of DBT-SRF.
Availability of data and materials
Not applicable.
Authors' contributions
RKS and DV conceptualized the manuscript. SB wrote the manuscript with input from RKS and DV. RKS and DV critically reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Klagsbrun M, D'Amore PA. Regulators of angiogenesis. Ann Review Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Patel-Hett S, D'Amore PA. Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol. 2011;55:353–363. doi: 10.1387/ijdb.103213sp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff J. Cell adhesion and angiogenesis. J Clin Inves. 1997;99:373–376. doi: 10.1172/JCI119168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramjaun AR, Hodivala-Dilke K. The role of cell adhesion pathways in angiogenesis. Int J Biochem Cell Biol. 2009;41:521–530. doi: 10.1016/j.biocel.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Mignatti P, Rifkin DB. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein. 1996;49:117–137. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- 11.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 12.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/S0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 13.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. American journal of physiology. Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 14.Dusse LM, Carvalho MG, Getliffe K, Voegeli D, Cooper AJ, Lwaleed BA. Increased circulating thrombomodulin levels in pre-eclampsia. Clin Chim Acta. 2008;387:168–171. doi: 10.1016/j.cca.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Wada H, Minamikawa K, Wakita Y, Nakase T, Kaneko T, Ohiwa M, Tamaki S, Deguchi K, Shirakawa S, Hayashi T, et al. Increased vascular endothelial cell markers in patients with disseminated intravascular coagulation. Am J Hematol. 1993;44:85–88. doi: 10.1002/ajh.2830440203. [DOI] [PubMed] [Google Scholar]
- 16.Mori Y, Wada H, Okugawa Y, Tamaki S, Nakasaki T, Watanabe R, Gabazza EC, Nishikawa M, Minami N, Shiku H. Increased plasma thrombomodulin as a vascular endothelial cell marker in patients with thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Clin Appl Thromb Hemost. 2001;7:5–9. doi: 10.1177/107602960100700102. [DOI] [PubMed] [Google Scholar]
- 17.Stratton RJ, Pompon L, Coghlan JG, Pearson JD, Black CM. Soluble thrombomodulin concentration is raised in scleroderma associated pulmonary hypertension. Ann Rheum Dis. 2000;59:132–134. doi: 10.1136/ard.59.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohi Y, Ohashi M, Sugiyama M, Takase H, Sato K, Ueda R. Circulating thrombomodulin levels are related to latent progression of atherosclerosis in hypertensive patients. Hypertens Res. 2003;26:479–483. doi: 10.1291/hypres.26.479. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulos DP, Thomopoulos C, Mourouzis I, Kotrotsou A, Sanidas E, Papazachou U, Daskalaki M, Makris TK. Masked hypertension unfavourably affects haemostasis parameters. Blood Press. 2011;20:218–221. doi: 10.3109/08037051.2011.565551. [DOI] [PubMed] [Google Scholar]
- 20.Maia M, de Vriese A, Janssens T, Moons M, van Landuyt K, Tavernier J, Lories RJ, Conway EM. CD248 and its cytoplasmic domain: A therapeutic target for arthritis. Arthritis Rheum. 2010;62:3595–3606. doi: 10.1002/art.27701. [DOI] [PubMed] [Google Scholar]
- 21.Lax S, Hou TZ, Jenkinson E, Salmon M, MacFadyen JR, Isacke CM, Anderson G, Cunningham AF, Buckley CD. CD248/Endosialin is dynamically expressed on a subset of stromal cells during lymphoid tissue development, splenic remodeling and repair. FEBS Lett. 2007;581:3550–3556. doi: 10.1016/j.febslet.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 22.Jeon JW, Jung JG, Shin EC, Choi HI, Kim HY, Cho ML, Kim SW, Jang YS, Sohn MH, Moon JH, et al. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J Immunol. 2010;185:4921–4927. doi: 10.4049/jimmunol.0904011. [DOI] [PubMed] [Google Scholar]
- 23.Moosig F, Fahndrich E, Knorr-Spahr A, Böttcher S, Ritgen M, Zeuner R, Kneba M, Schröder JO. C1qRP (CD93) expression on peripheral blood monocytes in patients with systemic lupus erythematosus. Rheumatol Int. 2006;26:1109–1112. doi: 10.1007/s00296-006-0132-5. [DOI] [PubMed] [Google Scholar]
- 24.van der Net JB, Oosterveer DM, Versmissen J, Defesche JC, Yazdanpanah M, Aouizerat BE, Steyerberg EW, Malloy MJ, Pullinger CR, Kastelein JJ, Kane JP. Replication study of 10 genetic polymorphisms associated with coronary heart disease in a specific high-risk population with familial hypercholesterolemia. Eur Heart J. 2008;29:2195–2201. doi: 10.1093/eurheartj/ehn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drickamer K. Demonstration of carbohydrate-recognition activity in diverse proteins which share a common primary structure motif. Biochem Soci Trans. 1989;17:13–15. doi: 10.1042/bst0170013. [DOI] [PubMed] [Google Scholar]
- 26.Drickamer K. C-type lectin-like domains. Curr Opinion Struct Biol. 1999;9:585–590. doi: 10.1016/S0959-440X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 27.Drickamer K, Fadden AJ. Genomic analysis of C-type lectins. Biochem Soci Symp. 2002:59–72. doi: 10.1042/bss0690059. [DOI] [PubMed] [Google Scholar]
- 28.Zelensky AN, Gready JE. C-type lectin-like domains in Fugu rubripes. BMC Genomics. 2004;5:51. doi: 10.1186/1471-2164-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drickamer K. Evolution of Ca(2+)-dependent animal lectins. Prog Nucleic Acid Res Mol Biol. 1993;45:207–232. doi: 10.1016/S0079-6603(08)60870-3. [DOI] [PubMed] [Google Scholar]
- 30.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 31.Rho SS, Choi HJ, Min JK, Lee HW, Park H, Park H, Kim YM, Kwon YG. Clec14a is specifically expressed in endothelial cells and mediates cell to cell adhesion. Biochem Biophys Res Commun. 2011;404:103–108. doi: 10.1016/j.bbrc.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 32.Mura M, Swain RK, Zhuang X, Vorschmitt H, Reynolds G, Durant S, Beesley JF, Herbert JM, Sheldon H, Andre M, et al. Identification and angiogenic role of the novel tumor endothelial marker CLEC14A. Oncogene. 2012;31:293–305. doi: 10.1038/onc.2011.233. [DOI] [PubMed] [Google Scholar]
- 33.Du J, Yang Q, Luo L, Yang D. C1qr and C1qrl redundantly regulate angiogenesis in zebrafish through controlling endothelial Cdh5. Biochem Biophys Res Commun. 2017;483:482–487. doi: 10.1016/j.bbrc.2016.12.118. [DOI] [PubMed] [Google Scholar]
- 34.Masiero M, Simoes FC, Han HD, Snell C, Peterkin T, Bridges E, Mangala LS, Wu SY, Pradeep S, Li D, et al. A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis. Cancer Cell. 2013;24:229–241. doi: 10.1016/j.ccr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delcourt N, Quevedo C, Nonne C, Fons P, O'Brien D, Loyaux D, Diez M, Autelitano F, Guillemot JC, Ferrara P, et al. Targeted identification of sialoglycoproteins in hypoxic endothelial cells and validation in zebrafish reveal roles for proteins in angiogenesis. J Biol Chem. 2015;290:3405–3417. doi: 10.1074/jbc.M114.618611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ki MK, Jeoung MH, Choi JR, Rho SS, Kwon YG, Shim H, Chung J, Hong HJ, Song BD, Lee S. Human antibodies targeting the C-type lectin-like domain of the tumor endothelial cell marker clec14a regulate angiogenic properties in vitro. Oncogene. 2013;32:5449–5457. doi: 10.1038/onc.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TK, Park CS, Jang J, Kim MR, Na HJ, Lee K, Kim HJ, Heo K, Yoo BC, Kim YM, et al. Inhibition of VEGF-dependent angiogenesis and tumor angiogenesis by an optimized antibody targeting CLEC14a. Mol Oncol. 2018;12:356–372. doi: 10.1002/1878-0261.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanivan S, Maione F, Hein MY, Hernández-Fernaud JR, Ostasiewicz P, Giraudo E, Mann M. SILAC-based proteomics of human primary endothelial cell morphogenesis unveils tumor angiogenic markers. Mol Cell Proteomics. 2013;12:3599–3611. doi: 10.1074/mcp.M113.031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dev KK. Making protein interactions druggable: Targeting PDZ domains. Nat Rev Drug Discov. 2004;3:1047–1056. doi: 10.1038/nrd1578. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/S0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Ji G, Wu Y, Wan B, Yu L. LAMA4, highly expressed in human hepatocellular carcinoma from Chinese patients, is a novel marker of tumor invasion and metastasis. J Cancer Res Clin Oncol. 2008;134:705–714. doi: 10.1007/s00432-007-0342-6. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzon E, Colladel R, Andreuzzi E, Marastoni S, Todaro F, Schiappacassi M, Ligresti G, Colombatti A, Mongiat M. MULTIMERIN2 impairs tumor angiogenesis and growth by interfering with VEGF-A/VEGFR2 pathway. Oncogene. 2012;31:3136–3147. doi: 10.1038/onc.2011.487. [DOI] [PubMed] [Google Scholar]
- 43.Lugano R, Vemuri K, Yu D, Bergqvist M, Smits A, Essand M, Johansson S, Dejana E, Dimberg A. CD93 promotes beta1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J Clin Invest. 2018;128:3280–3297. doi: 10.1172/JCI97459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan KA, Naylor AJ, Khan A, Noy PJ, Mambretti M, Lodhia P, Athwal J, Korzystka A, Buckley CD, Willcox BE, et al. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene. 2017;36:6097–6108. doi: 10.1038/onc.2017.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noy PJ, Lodhia P, Khan K, Zhuang X, Ward DG, Verissimo AR, Bacon A, Bicknell R. Blocking CLEC14A-MMRN2 binding inhibits sprouting angiogenesis and tumour growth. Oncogene. 2015;34:5821–5831. doi: 10.1038/onc.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang J, Kim MR, Kim TK, Lee WR, Kim JH, Heo K, Lee S. CLEC14a-HSP70-1A interaction regulates HSP70-1A-induced angiogenesis. Sci Re. 2017;7:10666. doi: 10.1038/s41598-017-11118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noy PJ, Swain RK, Khan K, Lodhia P, Bicknell R. Sprouting angiogenesis is regulated by shedding of the C-type lectin family 14, member A (CLEC14A) ectodomain, catalyzed by rhomboid-like 2 protein (RHBDL2) FASEB J. 2016;30:2311–2323. doi: 10.1096/fj.201500122R. [DOI] [PubMed] [Google Scholar]
- 48.Lee S, Rho SS, Park H, Park JA, Kim J, Lee IK, Koh GY, Mochizuki N, Kim YM, Kwon YG. Carbohydrate-binding protein CLEC14A regulates VEGFR-2- and VEGFR-3-dependent signals during angiogenesis and lymphangiogenesis. J Clin Invest. 2017;127:457–471. doi: 10.1172/JCI85145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomäki A, Aranda E, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki K, Kusumoto H, Deyashiki Y, Nishioka J, Maruyama I, Zushi M, Kawahara S, Honda G, Yamamoto S, Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987;6:1891–1897. doi: 10.1002/j.1460-2075.1987.tb02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107–125. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 52.Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985;101:363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soff GA, Jackman RW, Rosenberg RD. Expression of thrombomodulin by smooth muscle cells in culture: Different effects of tumor necrosis factor and cyclic adenosine monophosphate on thrombomodulin expression by endothelial cells and smooth muscle cells in culture. Blood. 1991;77:515–518. [PubMed] [Google Scholar]
- 54.McCachren SS, Diggs J, Weinberg JB, Dittman WA. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood. 1991;78:3128–3132. [PubMed] [Google Scholar]
- 55.Calnek DS, Grinnell BW. Thrombomodulin-dependent anticoagulant activity is regulated by vascular endothelial growth factor. Exp Cell Res. 1998;238:294–298. doi: 10.1006/excr.1997.3812. [DOI] [PubMed] [Google Scholar]
- 56.Huang HC, Shi GY, Jiang SJ, Shi CS, Wu CM, Yang HY, Wu HL. Thrombomodulin-mediated cell adhesion: Involvement of its lectin-like domain. J Biol Chem. 2003;278:46750–46759. doi: 10.1074/jbc.M305216200. [DOI] [PubMed] [Google Scholar]
- 57.Suehiro T, Shimada M, Matsumata T, Taketomi A, Yamamoto K, Sugimachi K. Thrombomodulin inhibits intrahepatic spread in human hepatocellular carcinoma. Hepatology. 1995;21:1285–1290. doi: 10.1002/hep.1840210511. [DOI] [PubMed] [Google Scholar]
- 58.Tabata M, Sugihara K, Yonezawa S, Yamashita S, Maruyama I. An immunohistochemical study of thrombomodulin in oral squamous cell carcinoma and its association with invasive and metastatic potential. J Oral Pathol Med. 1997;26:258–264. doi: 10.1111/j.1600-0714.1997.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 59.Hsu YY, Shi GY, Wang KC, Ma CY, Cheng TL, Wu HL. Thrombomodulin promotes focal adhesion kinase activation and contributes to angiogenesis by binding to fibronectin. Oncotarget. 2016;7:68122–68139. doi: 10.18632/oncotarget.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng X, Ueda H, Zhou H, Stokol T, Shen TL, Alcaraz A, Nagy T, Vassalli JD, Guan JL. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc Rese. 2004;64:421–430. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 62.Kao YC, Wu LW, Shi CS, Chu CH, Huang CW, Kuo CP, Sheu HM, Shi GY, Wu HL. Downregulation of thrombomodulin, a novel target of Snail, induces tumorigenesis through epithelial-mesenchymal transition. Mol Cell Biol. 2010;30:4767–4785. doi: 10.1128/MCB.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo CH, Chen PK, Chang BI, Sung MC, Shi CS, Lee JS, Chang CF, Shi GY, Wu HL. The recombinant lectin-like domain of thrombomodulin inhibits angiogenesis through interaction with Lewis Y antigen. Blood. 2012;119:1302–1313. doi: 10.1182/blood-2011-08-376038. [DOI] [PubMed] [Google Scholar]
- 64.Hosaka Y, Higuchi T, Tsumagari M, Ishii H. Inhibition of invasion and experimental metastasis of murine melanoma cells by human soluble thrombomodulin. Cancer Lett. 2000;161:231–240. doi: 10.1016/S0304-3835(00)00617-0. [DOI] [PubMed] [Google Scholar]
- 65.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A, Weitz JI, Weiler H, Hellings PW, Schaeffer P, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamatake M, Ishida T, Mitsudomi T, Akazawa K, Sugimachi K. Prognostic value and clinicopathological correlation of thrombomodulin in squamous cell carcinoma of the human lung. Clin Cancer Res. 1996;2:763–766. [PubMed] [Google Scholar]
- 67.Tezuka Y, Yonezawa S, Maruyama I, Matsushita Y, Shimizu T, Obama H, Sagara M, Shirao K, Kusano C, Natsugoe S, et al. Expression of thrombomodulin in esophageal squamous cell carcinoma and its relationship to lymph node metastasis. Cancer Res. 1995;55:4196–4200. [PubMed] [Google Scholar]
- 68.Hanly AM, Redmond M, Winter DC, Brophy S, Deasy JM, Bouchier-Hayes DJ, Kay EW. Thrombomodulin expression in colorectal carcinoma is protective and correlates with survival. Br J Cancer. 2006;94:1320–1325. doi: 10.1038/sj.bjc.6603098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Weiler-Guettler H, Chen J, Wilhelm O, Deng Y, Qiu F, Nakagawa K, Klevesath M, Wilhelm S, Böhrer H, et al. Thrombomodulin modulates growth of tumor cells independent of its anticoagulant activity. J Clin Invest. 1998;101:1301–1309. doi: 10.1172/JCI925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindahl AK, Boffa MC, Abildgaard U. Increased plasma thrombomodulin in cancer patients. Thromb Haemost. 1993;69:112–114. doi: 10.1055/s-0038-1651564. [DOI] [PubMed] [Google Scholar]
- 71.Salmaggi A, Eoli M, Frigerio S, Ciusani E, Silvani A, Boiardi A. Circulating intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and plasma thrombomodulin levels in glioblastoma patients. Cancer Lett. 1999;146:169–172. doi: 10.1016/S0304-3835(99)00255-4. [DOI] [PubMed] [Google Scholar]
- 72.Hsu YY, Shi GY, Kuo CH, Liu SL, Wu CM, Ma CY, Lin FY, Yang HY, Wu HL. Thrombomodulin is an ezrin-interacting protein that controls epithelial morphology and promotes collective cell migration. FASEB J. 2012;26:3440–3452. doi: 10.1096/fj.12-204917. [DOI] [PubMed] [Google Scholar]
- 73.Zheng N, Huo Z, Zhang B, Meng M, Cao Z, Wang Z, Zhou Q. Thrombomodulin reduces tumorigenic and metastatic potential of lung cancer cells by up-regulation of E-cadherin and down-regulation of N-cadherin expression. Biochem Biophys Res Commun. 2016;476:252–259. doi: 10.1016/j.bbrc.2016.05.105. [DOI] [PubMed] [Google Scholar]
- 74.Shi CS, Shi GY, Chang YS, Han HS, Kuo CH, Liu C, Huang HC, Chang YJ, Chen PS, Wu HL. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation. 2005;111:1627–1636. doi: 10.1161/01.CIR.0000160364.05405.B5. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Pan B, Honda G, Wang X, Hashimoto Y, Ohkawara H, Xu K, Zeng L, Ikezoe T. Cytoprotective and pro-angiogenic functions of thrombomodulin are preserved in the C loop of the fifth epidermal growth factor-like domain. Haematologica. 2018;103:1730–1740. doi: 10.3324/haematol.2017.184481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuo CH, Sung MC, Chen PK, Chang BI, Lee FT, Cho CF, Hsieh TT, Huang YC, Li YH, Shi GY, et al. FGFR1 mediates recombinant thrombomodulin domain-induced angiogenesis. Cardiovasc Res. 2015;105:107–117. doi: 10.1093/cvr/cvu239. [DOI] [PubMed] [Google Scholar]
- 77.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/S1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 78.Malarstig A, Silveira A, Wagsater D, Öhrvik J, Bäcklund A, Samnegård A, Khademi M, Hellenius ML, Leander K, Olsson T, et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J Intern Med. 2011;270:229–236. doi: 10.1111/j.1365-2796.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 79.McGreal EP, Ikewaki N, Akatsu H, Morgan BP, Gasque P. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. J Immunol. 2002;168:5222–5232. doi: 10.4049/jimmunol.168.10.5222. [DOI] [PubMed] [Google Scholar]
- 80.Bohlson SS, Silva R, Fonseca MI, Tenner AJ. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol. 2005;175:1239–1247. doi: 10.4049/jimmunol.175.2.1239. [DOI] [PubMed] [Google Scholar]
- 81.Nepomuceno RR, Tenner AJ. C1qRP, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J Immunol. 1998;160:1929–1935. [PubMed] [Google Scholar]
- 82.Langenkamp E, Zhang L, Lugano R, Huang H, Elhassan TE, Georganaki M, Bazzar W, Lööf J, Trendelenburg G, Essand M, et al. Elevated expression of the C-type lectin CD93 in the glioblastoma vasculature regulates cytoskeletal rearrangements that enhance vessel function and reduce host survival. Cancer Res. 2015;75:4504–4516. doi: 10.1158/0008-5472.CAN-14-3636. [DOI] [PubMed] [Google Scholar]
- 83.Bao L, Tang M, Zhang Q, You B, Shan Y, Shi S, Li L, Hu S, You Y. Elevated expression of CD93 promotes angiogenesis and tumor growth in nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2016;476:467–474. doi: 10.1016/j.bbrc.2016.05.146. [DOI] [PubMed] [Google Scholar]
- 84.Tosi GM, Caldi E, Parolini B, Toti P, Neri G, Nardi F, Traversi C, Cevenini G, Marigliani D, Nuti E, et al. CD93 as a potential target in neovascular age-related macular degeneration. J Cell Physiol. 2017;232:1767–1773. doi: 10.1002/jcp.25689. [DOI] [PubMed] [Google Scholar]
- 85.Dieterich LC, Mellberg S, Langenkamp E, Zhang L, Zieba A, Salomäki H, Teichert M, Huang H, Edqvist PH, Kraus T, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFbeta2 in vascular abnormalization. J Pathol. 2012;228:378–390. doi: 10.1002/path.4072. [DOI] [PubMed] [Google Scholar]
- 86.Greenlee MC, Sullivan SA, Bohlson SS. Detection and characterization of soluble CD93 released during inflammation. Inflamm Res. 2009;58:909–919. doi: 10.1007/s00011-009-0064-0. [DOI] [PubMed] [Google Scholar]
- 87.Petrenko O, Beavis A, Klaine M, Kittappa R, Godin I, Lemischka IR. The molecular characterization of the fetal stem cell marker AA4. Immunity. 1999;10:691–700. doi: 10.1016/S1074-7613(00)80068-0. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M, Bohlson SS, Dy M, Tenner AJ. Modulated interaction of the ERM protein, moesin, with CD93. Immunology. 2005;115:63–73. doi: 10.1111/j.1365-2567.2005.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galvagni F, Nardi F, Maida M, Bernardini G, Vannuccini S, Petraglia F, Santucci A, Orlandini M. CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration adhesion and migration. Oncotarget. 2016;7:10090–10103. doi: 10.18632/oncotarget.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galvagni F, Nardi F, Spiga O, Trezza A, Tarticchio G, Pellicani R, Andreuzzi E, Caldi E, Toti P, Tosi GM, et al. Dissecting the CD93-Multimerin 2 interaction involved in cell adhesion and migration of the activated endothelium. Matrix Biol. 2017;64:112–127. doi: 10.1016/j.matbio.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Kao YC, Jiang SJ, Pan WA, Wang KC, Chen PK, Wei HJ, Chen WS, Chang BI, Shi GY, Wu HL. The epidermal growth factor-like domain of CD93 is a potent angiogenic factor. PLoS One. 2012;7:e51647. doi: 10.1371/journal.pone.0051647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orlandini M, Galvagni F, Bardelli M, Rocchigiani M, Lentucci C, Anselmi F, Zippo A, Bini L, Oliviero S. The characterization of a novel monoclonal antibody against CD93 unveils a new antiangiogenic target. Oncotarget. 2014;5:2750–2760. doi: 10.18632/oncotarget.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi HP, Garin-Chesa P, Park JE, Rettig WJ, Lenter MC. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276:7408–7414. doi: 10.1074/jbc.M009604200. [DOI] [PubMed] [Google Scholar]
- 94.Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci USA. 1992;89:10832–10836. doi: 10.1073/pnas.89.22.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bagley RG, Weber W, Rouleau C, Yao M, Honma N, Kataoka S, Ishida I, Roberts BL, Teicher BA. Human mesenchymal stem cells from bone marrow express tumor endothelial and stromal markers. Int J Oncol. 2009;34:619–627. doi: 10.3892/ijo_00000187. [DOI] [PubMed] [Google Scholar]
- 96.Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D, Augustin HG. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172:486–494. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Becker R, Lenter MC, Vollkommer T, Boos AM, Pfaff D, Augustin HG, Christian S. Tumor stroma marker endosialin (Tem1) is a binding partner of metastasis-related protein Mac-2 BP/90K. FASEB J. 2008;22:3059–3067. doi: 10.1096/fj.07-101386. [DOI] [PubMed] [Google Scholar]
- 98.Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E, Sass P, Nicolaides NC, Grasso L, Zhou Y. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci USA. 2007;104:17965–17970. doi: 10.1073/pnas.0705647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, Vogelstein B, St Croix B, Kinzler KW, Huso DL. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci USA. 2006;103:3351–3356. doi: 10.1073/pnas.0511306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maia M, DeVriese A, Janssens T, Moons M, Lories RJ, Tavernier J, Conway EM. CD248 facilitates tumor growth via its cytoplasmic domain. BMC Cancer. 2011;11:162. doi: 10.1186/1471-2407-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeo M, Park HJ, Kim DK, Kim YB, Cheong JY, Lee KJ, Cho SW. Loss of SM22 is a characteristic signature of colon carcinogenesis and its restoration suppresses colon tumorigenicity in vivo and in vitro. Cancer. 2010;116:2581–2589. doi: 10.1002/cncr.25003. [DOI] [PubMed] [Google Scholar]
- 102.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17:145–159. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 103.Ohradanova A, Gradin K, Barathova M, Zatovicova M, Holotnakova T, Kopacek J, Parkkila S, Poellinger L, Pastorekova S, Pastorek J. Hypoxia upregulates expression of human endosialin gene via hypoxia-inducible factor 2. Br J Cancer. 2008;99:1348–1356. doi: 10.1038/sj.bjc.6604685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist. 2015;20:660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.