Fig. 10.
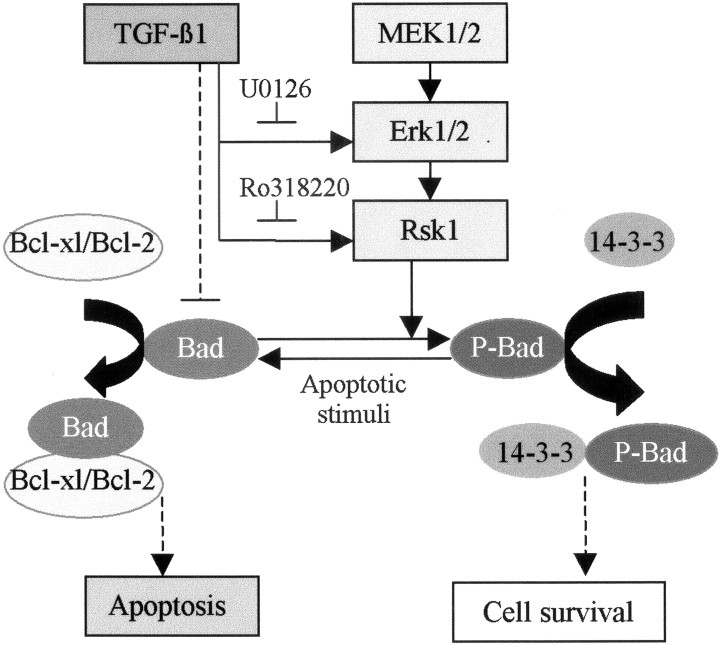
Scheme summarizing the major findings of this study. Either adenovirus-mediated overexpression of TGF-β1 in mouse brain or administration of TGF-β1 to the cultured hippocampal cells caused an activation of Erk1/2 and Rsk1, consistently accompanied by an increase in phosphorylation of Bad (Ser112). TGF-β1-mediated activation of MAPK, phosphorylation of Bad, and an antiapoptotic effect in cultured hippocampal cells were abolished by MEK/Erk1/2 and Rsk1 inhibitors, indicating a tight association between MAPK activation and Bad phosphorylation, as well as a requirement of MAPK pathway for the antiapoptotic activity of TGF-β1. It is proposed that the increase in phosphorylation of Bad may lead to a dissociation of Bcl-2 and Bcl-xl from the complex of Bad/Bcl-xl or Bad/Bcl-2, which in turn interrupts multiple apoptotic cascades, including inhibition of caspase-3 activation. These effects, together with the inhibitory effect of TGF-β1 on the lesion-mediated increase in Bad level, may crucially contribute to the neuroprotective activity of TGF-β1, as evidenced by decrease in DNA degradation and ischemic lesions, as well as the improvement of the neurological outcome after cerebral ischemia.