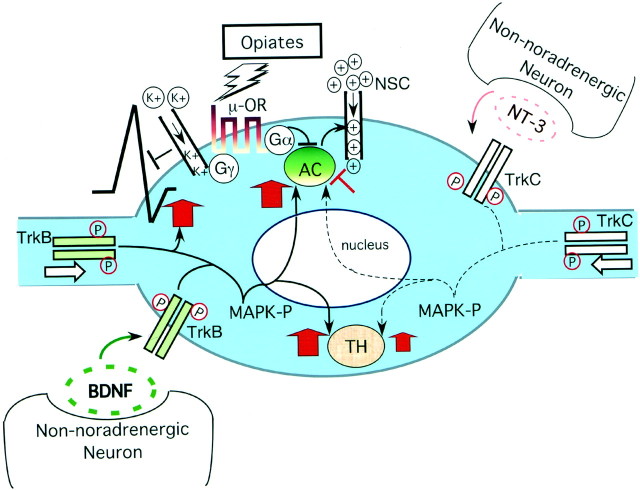
Fig. 8.
A model whereby neurotrophins regulate opiate-induced adaptations in noradrenergic neurons. Ligand-activated opiate receptors, through GαI subunits, inhibit adenylyl cyclase (AC) activity, and this results in decreased depolarization currents through nonselective cation channels (NSC). Opiate receptors reduce neuronal activity further through Gβ/γ subunits that activate potassium channels and inhibit voltage-sensitive calcium channels (Williams et al., 2001). In the drug-adapted state, BDNF offsets opiate-mediated neuronal inhibition by increasing levels of ACs and by restoring neuronal firing rates. NT-3, which plays a less dominant role in the noradrenergic system, appears to have a weak inhibitory effect on the cAMP pathway after chronic opiate exposure (Akbarian et al., 2001). Both BDNF and NT-3 increase TH expression in the drug-adapted state. It is yet unclear whether the neurotrophins regulate TH and AC expression at the level of gene transcription and whether mitogen-activated protein kinase (MAPK) pathways are involved. Both BDNF and NT-3 are released by non-noradrenergic neurons and activate TrkB (BDNF) and TrkC (NT-3) receptors by dimerization and phosphorylation either at the somal membrane or in distal processes of noradrenergic neurons.