Fig. 9.
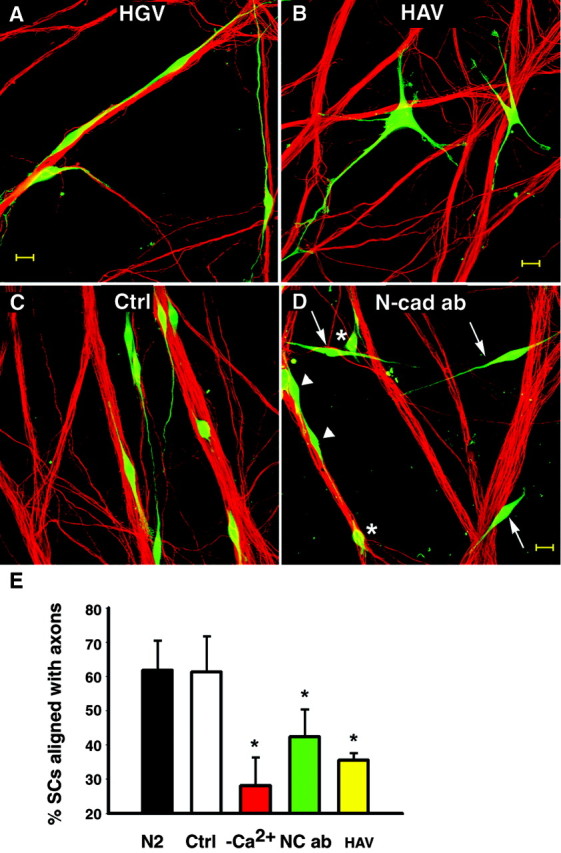
N-cadherin function-blocking agents decrease the number of SCs aligning to axons. A, B, Cell Tracker-labeled SCs (green) were cocultured with DRG neurons in the presence of 0.5 mg/ml of the cyclic pentapeptides HGV (0.5 mg/ml) or HAV (0.5 mg/ml). After 4 hr, cultures were fixed and axons were stained for neurofilament M (red). A, Spindle-shaped SCs are seen with their extensions aligned to axons in cultures treated with the control peptide (HGV). B, In the presence of blocking peptide HAV, SCs display multiple processes that failed to align with axons. Scale bar, 20 μm. C,D, SCs (Cell Tracker, green) were cocultured with DRG neurons for 24 hr in defined medium (ctrl; N2) or the presence of N-cadherin function-blocking guinea pig antibody (Ncad ab; gp1260 IgG, 1:100). C, In control cultures (N2), most SCs show processes well aligned to axon bundles. Both N-cadherin-blocking antibodies (L7 IgG and gp1260) were used to treat SC–DRG cocultures.D, A coculture treated with N-cadherin-blocking antibody (here gp1260 IgG) shows SCs that failed to associate with axons (arrows) as well as SCs with short or no processes (∗) and some SCs with axon-aligned processes (arrowheads). Scale bar, 20 μm. E, Plotted are average percentages of SCs aligned with axons. Error bars represent SDs from three cultures of each condition. Significantly fewer SCs align to axons in HAV as well as N-cadherin-blocking antibody (here data from L7 IgG)-treated cocultures compared with untreated (N2) and controls treated (average of HGV, rabbit IgG, and L4 IgG combined;p < 0.01, Tukey–Kramer multiple comparisons). There was no significant difference between HAV and low Ca2+ (Tukey–Kramer, multiple comparisons).
