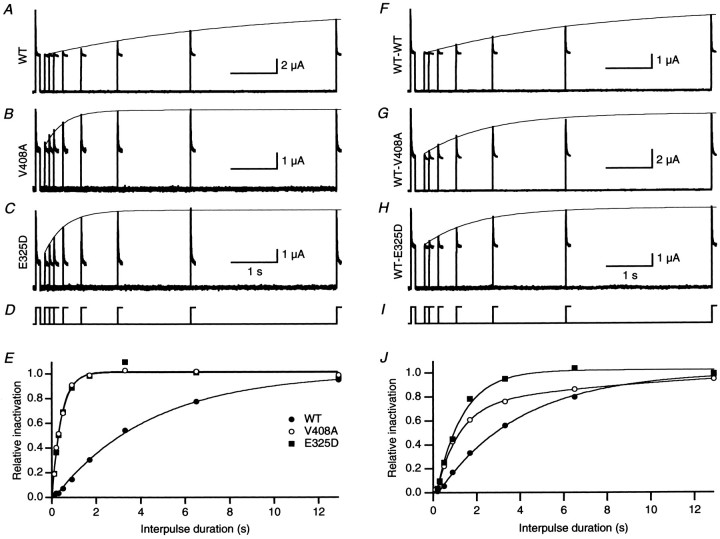
Fig. 3.
EA1 mutations V408A and E325D accelerate recovery from inactivation induced by hKvβ1. A–C, Recovery from inactivation was determined using a double-pulse protocol to 40 mV separated by an interpulse interval of 0.1–13 sec at a holding potential of −80 mV (D). Superimposed current traces recorded for interpulse intervals from 0.1 to 13 sec for homomeric WT, V408A, and E325D. A single exponential (continuous line) determined in E was overlaid with the envelope of peaks of the second pulse. E, The relative amount of inactivation normalized by the first pulse plotted as a function of interpulse duration for homomeric WT, V408A, and E325D fromA–C. The data were fit with a single-exponential function, yielding a time constant of recovery of 4.1, 0.4, and 0.4 sec for WT (filled circles), V408A (open circles), and E325D (filled squares), respectively. F–I, Recovery from inactivation of heteromeric channels formed by WT-WT, WT-V408A, and WT-E325D coexpressed with hKvβ1. J, Plot of relative recovery from inactivation versus interpulse duration for WT-WT (filled circles), WT-V408A (open circles), and WT-E325D (filled squares). The time constants of recovery determined from single exponentials were 3.3, 1.1, and 1.2 sec, respectively.