Abstract
Human hand dexterity depends on the ability to move digits independently and to combine these movements in various coordinative patterns. It is well established that the primary motor cortex (M1) is important for skillful digit actions but less is known about the role played by the nonprimary motor centers. Here we use functional magnetic resonance imaging to examine the hypothesis that nonprimary motor areas and the posterior parietal cortex are strongly activated when healthy humans move the right digits in a skillful coordination pattern involving relatively independent digit movements. A task in which flexion of the thumb is accompanied by extension of the fingers and vice versa, i.e., a learned “nonsynergistic” coordination pattern, is contrasted with a task in which all digits flex and extend simultaneously in an innate synergistic coordination pattern (opening and closing the fist). The motor output is the same in the two conditions. Thus, the difference when contrasting the nonsynergistic and synergistic tasks represents the requirement to fractionate the movements of the thumb and fingers and to combine these movements in a learned coordinative pattern. The supplementary (and cingulate) motor area, the bilateral dorsal premotor area, the bilateral lateral cerebellum, the bilateral cortices of the postcentral sulcus, and the left intraparietal cortex showed stronger activity when the subjects made the nonsynergistic flexion–extension movements of the digits than when the synergistic movements were made. These results suggest that the human neural substrate for skillful digit movement includes a sensorimotor network of nonprimary frontoparietal areas and the cerebellum that, in conjunction with M1, control the movements of the digits.
Keywords: supplementary motor area, premotor cortex, cerebellum, posterior parietal cortex, manual dexterity, hand posture
Hand dexterity in humans depends on the ability to move the fingers and thumb independently. Independent movements of the digits are used in many hand maneuvers, such as the manipulation of objects, tool usage, gesticulation, and when playing musical instruments. The critical aspect of independent digit actions is that one or more of the digits moves relatively independently from the movements and postures of the other digits. The capability to make independent digit movements provides great flexibility and the possibility to combine the movements of the digits in various coordinative patterns. These dexterous actions differ markedly from those used in phylogenetically older movements when all digits are moved together in simple “synergistic” coordination patterns, e.g., when clenching the fist or grasping an object in a palmar grasp (power grip) (Napier, 1956, 1961). The ability to perform synergistic finger movements is innate, and the palmar grasp is already present in its reflexive form in the newborn infant (Twitchell, 1970). It is also the first voluntary grasp movement to develop (Halverson, 1931). After brain damage in adults (e.g., stroke), it is a common clinical observation that the synergistic movement patterns, including the palmar grasp, are the first to recover. In nonhuman primates, lesions of the primary motor cortex (M1) and the corticospinal tract impair the ability to perform independent finger movements, whereas synergistic whole hand movements are minimally influenced (Lawrence and Kuypers, 1968; Passingham et al., 1983; Rouiller et al., 1998). It is evident that the corticospinal neurons in the primary motor cortex play a critical role in the production of independent digit actions (Kuypers, 1981; Porter and Lemon, 1993; Lemon et al., 1998). These observations suggest that dexterous hand actions in which the digits move independently require another type of cortical control than simpler synergistic movements.
In this study we examine the hypothesis that nonprimary motor and posterior parietal areas are more strongly engaged in the control of skillful movement of the digits, in which the digits flex and extend relatively independently (“nonsynergistic movement”), than in the production of synergistic movement. The rationale for the hypothesis was, in addition to the existing neurophysiological and developmental studies referred to above, the observation that impaired performance of various independent digit actions occurs in human subjects after brain lesions affecting nonprimary motor areas [supplementary motor area (SMA), lateral premotor cortex (PM), and the posterior parietal cortex (Kleist, 1907; Luria, 1966; Freund, 1987; Leiguarda and Marsden, 2000)]. Furthermore, several functional imaging studies have shown increased activity in the primary motor cortex, SMA, PM, and other nonprimary frontoparietal areas when subjects generate various skilled hand postures involving independent digit actions (Roland et al., 1980;Colebatch et al., 1991; Passingham, 1993; Roland and Zilles, 1996;Sadato et al., 1996; Binkofski et al., 1999; Rijntjes et al., 1999;Ehrsson et al., 2001). However, simple synergistic movements (like opening and closing the fist or making a palmar grasp) also activate the primary motor cortex and similar nonprimary frontal motor areas (including SMA and PM) and the posterior parietal cortex (Olesen, 1971;Colebatch et al., 1991; Roland, 1993; Ehrsson et al., 2000). Yet no study has compared nonsynergistic actions with synergistic actions that are matched in terms of the motor output (that is, the same muscle groups are involved and there is similar velocity, amplitude, and frequency of the movements), and therefore it remains unclear whether the cortical centers are more strongly activated when nonsynergistic digit movements are produced than when synergistic ones are generated.
Here, we compare a task in which the subjects flex and extend all digits simultaneously (making synergistic movement) with a task in which flexion of the thumb is accompanied by extension of the fingers and vice versa (making nonsynergistic movement). The key point of this experimental design is that the motor output is matched. This means that a direct comparison between the two tasks (nonsynergistic versus synergistic movement) will reflect the neural control mechanisms specific to nonsynergistic movement of the digits.
MATERIALS AND METHODS
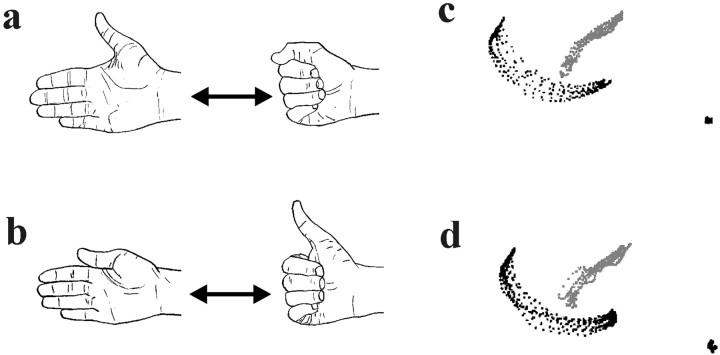
Tasks. We compared the “synergistic digit movement” task, with simultaneous flexion–extension movements of the fingers and thumb (Fig.1a,c), and the “nonsynergistic digit movement” task in which flexion of the thumb was accompanied by extension of the fingers and vice versa (Fig.1b,d). In both tasks subjects alternated between two natural hand postures paced by auditory signals (0.5 Hz). A rest condition, in which the subject held the hand in a relaxed position (anatomical resting position) and listened to the auditory signals (0.5 Hz), was also included.
Fig. 1.
A schematic presentation of the synergistic (a) and nonsynergistic (b) digit movement tasks. In the synergistic task, thumb and fingers are flexed and extended together. In the nonsynergistic task, the thumb is flexed when the fingers are extended, and vice versa. The right hand is used in both tasks. The repetitive movement trajectories of the thumb (gray) and index finger (black), recorded after the training session and before the image recording, are displayed from one subject performing the synergistic movement (c) and the nonsynergistic movements (d) (1/60 sec between each point; scale 1:7).
Subjects and performance. Eight right-handed (mean laterality quotient +92, range +68 to +100) male subjects (21–33 years) with no history of neurological disease participated in the study (Oldfield, 1971). All subjects gave their informed consent, and the ethical committee of the Karolinska Hospital approved the study.
The subjects rehearsed the movement tasks for 30–40 min before the brain scanning. After 5–10 min of training, the subjects were able to keep up a conversation and do simple mental calculations while performing the tasks (at 0.5 Hz), which suggests that the movements had been well learned. We then continued with training for ∼30 min to assure that the movements were overlearned. The slow frequency of the movements (0.5 Hz) ensured that the tasks were simple to perform and avoided muscular fatigue.
We recorded the movement amplitude and movement velocity of the thumb and index finger with a quantitative motion analysis system (MacReflex, Sävdalen, Sweden). The subjects had their arm and hand in the same position (supine) as they had during the brain scanning. Reflective markers were attached to the tips of the thumb and index finger and to the wrist. The subjects performed each of the tasks for a 30 sec period, during which time motion data were collected (using a sampling frequency of 50 Hz) that were stored and analyzed on a computer. During the brain scanning, the movement performance was monitored with a digital video camera assuring that the movements were performed as requested (no mistakes were observed in either task).
Brain scanning. A 1.5 T General Electrics scanner with head-coil provided T1-weighted anatomical images (3D-SPGR) and functional T2*-weighted echo planar images with blood oxygenation level-dependent (BOLD) contrast [64 × 64 matrix; 3.4 × 3.4 mm; echo time (TE) = 60 msec]. A functional image volume comprised 21 slices of 7 mm thickness, which ensured that the whole brain was within the field of view. During the experiments the subjects rested comfortably in a supine position in the magnetic resonance scanner. The extended right arm was oriented in a relaxed supine position parallel to the trunk. It was supported proximal to the wrist to minimize movement. The subjects were blindfolded. For each subject functional images were collected in four 450 sec runs [repetition time (TR) = 5 sec], which meant that a total of 360 functional volumes was collected for each subject. For each run, the two motor tasks and a rest condition (the hand relaxed) were performed alternately in 30 sec periods.
We used the Statistical Parametric Mapping software (SPM99;http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Cognitive Neurology, London) to process the images. The functional images were realigned to correct for head movements (and reformatted to isometric voxels using linear sinc interpolation). Then, the functional images were coregistered with each subject's anatomical magnetic resonance (MR) image and normalized (linear and nonlinear transformations) to the standard coordinate system of Talaraich and Tournoux (1988) using the Montreal Neurological Institute (MNI) reference brain (Evans et al., 1994; Ashburner and Friston, 1997). The images were scaled to 100 to eliminate the effects of global changes in the signal, and a high-pass filter (cutoff frequency 0.00556 Hz) was used to remove low-frequency drifts and fluctuations of the signal. The functional images were spatially smoothed with a 9 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel and smoothed in time by a 4 sec FWHM Gaussian kernel. Data were analyzed with the program SPM99. We fitted a linear regression model (general linear model) to the pooled data from all subjects to increase the sensitivity of the analysis (fixed effects model) (Friston et al., 1995; Holmes et al., 1997). Each task was modeled with a boxcar function that had been filtered with the standard SPM99 hemodynamic response function. The linear contrasts of the parameter estimates generated statistical images of tstatistics. These statistical images were first thresholded att = 3.72 (p < 0.0001 at each voxel, without correction for multiple comparisons). Only clusters of active voxels and local maximas of activity (peaks) are reported that correspond to a p < 0.05 after a correction for the number of multiple comparisons in the whole-brain space using tests based on the Gaussian Random Field Theory (Poline et al., 1997). For the brain regions that showed activity when we contrasted pairs of digit movement tasks, we only report voxels that were active as compared with the rest condition (using inclusive masking; for each voxel t = 3.09, corresponding to p = 0.001 without correction for multiple comparisons). In a complementary analysis, we also confirmed that the results obtained in the group analysis were consistent with the activation maps obtained from the majority of the individual subjects (see Table 2).
Table 2.
Activity specific to the nonsynergistic digit movement (flexion/extension)
| Functional region, anatomical region | Talaraich coordinates (MNI brain) | BOLD signal increase | Number of subjects | Cluster volume | tvalue (peak) | p value (corrected) | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Synergistic | Nonsynergistic | |||||
| Left PMD | −36 | −8 | 52 | 0.33 ± 0.03 | 0.51 ± 0.03 | 72-a | 1800 | 6.37 | <0.001 |
| Left SMA/CMA2-b | −4 | −4 | 52 | 0.88 ± 0.05 | 1.12 ± 0.05 | 72-a | 2370 | 5.32 | <0.001 |
| Left intraparietal sulcus (anterior part) | −40 | −40 | 60 | 0.64 ± 0.05 | 0.82 ± 0.05 | 72-a | 3390 | 4.87 | 0.01 |
| Left postcentral sulcus | −48 | −36 | 48 | 0.66 ± 0.05 | 0.88 ± 0.05 | 7 | (Same cluster) | 4.66 | 0.025 |
| Right PMD | 36 | −8 | 52 | 0.14 ± 0.03 | 0.23 ± 0.03 | 7 | 1150 | 6.84 | <0.001 |
| Right postcentral sulcus | 40 | −32 | 44 | 0.09 ± 0.03 | 0.26 ± 0.03 | 52-c | 580 | 5.06 | 0.004 |
| Right cerebellar hemisphere (lobule VI)2-d | 24 | −56 | −24 | 0.96 ± 0.04 | 1.12 ± 0.04 | 72-a | 770 | 4.91 | 0.009 |
| Left anterior cerebellar hemisphere (lobule VI)2-d | −24 | −56 | −24 | 0.31 ± 0.03 | 0.47 ± 0.04 | 8 | 1790 | 6.51 | <0.001 |
Significant increases in BOLD contrast signal (p< 0.05 corrected for multiple comparisons) obtained by contrasting the nonsynergistic digit movement task versus the synergistic digit movement task. The p values correspond to the test for peak height. The cluster volumes (mm3), number of individual subjects (of the eight) that showed a statistical trend for the activity to increase (p < 0.05 uncorrected), and the adjusted BOLD contrast values detected in the two tasks compared with the resting condition are presented (% signal ± SE). All regions showed significantly stronger activity during the non-synergistic spreading movement than for the synergistic spreading movement (at each voxel p < 0.05 after a correction for multiple comparisons).
The eighth subject showed a weaker activation in these areas (p < 0.20).
The peak of the activation was located in SMA, and an additional peak, which did not reach statistical significance, was located in the upper bank of the cingulate sulcus (CMA:x = −8, y = 4, z = 40;t = 4.12; corrected p value = 0.192).
A sixth subject showed a weaker increase in activity in this areas (p < 0.10).
The localization of lobule VI refers to the Schmahman atlas of human cerebellum in standard space (Schmahman et al., 1999).
RESULTS
Performance
Before brain scanning, subjects trained for the tasks until they could produce the requested movements with ease (see Materials and Methods). We ensured that the amplitude and velocity of the movements in the two tasks were equal by recording the movements with a motion analysis system outside the MR scanner (Table1). There was no significant difference in the amplitude (trajectory length) and velocity of the movements (or the intra-subject variability of these parameters) for the movements of the thumb and index finger between the tasks (p> 0.05, paired t test, without correction for multiple comparisons).
Table 1.
Kinematic analysis of flexion/extension movements
| Movements | Synergistic movement | Nonsynergistic movement |
|---|---|---|
| Mean ± SD (mean intrasubject SD ± SD) | Mean ± SD (mean intrasubject SD ± SD) | |
| Thumb amplitude (cm) | 9.8 ± 1.2 (1.41 ± 0.50) | 10.3 ± 2.6 (1.71 ± 0.67) |
| Thumb extension velocity (cm/sec) | 39.5 ± 10.5 (5.65 ± 2.02) | 40.9 ± 8.8 (6.85 ± 2.70) |
| Thumb flexion velocity (cm/sec) | 38.7 ± 11.3 (6.67 ± 2.36) | 43.6 ± 9.9 (8.52 ± 2.28) |
| Index amplitude (cm) | 15.0 ± 2.6 (1.87 ± 0.65) | 15.7 ± 2.1 (2.25 ± 1.13) |
| Index extension velocity (cm/sec) | 74.4 ± 23.8 (7.49 ± 2.62) | 76.8 ± 20.5 (9.79 ± 4.52) |
| Index flexion velocity (cm/sec) | 70.2 ± 22.6 (7.69 ± 1.86) | 78.6 ± 20.1 (9.54 ± 4.68) |
Group average (and SD) of the kinematic data from the eight subjects who participated in the fMRI scanning. In each person, 10 consecutive movement cycles were recorded and analyzed at the end of the training (immediately before the fMRI was conducted). There were no significant differences between any of the parameters (or the SD) in the two conditions (p > 0.05, paired ttests, without correction for multiple comparisons).
Brain activation
Several brain regions showed stronger activity during the nonsynergistic movement than during the synergistic movement, including the bilateral dorsal premotor area (PMD), the SMA/cingulate motor area (CMA) (with the peak of the activation located in the left SMA and the cluster of active voxels extending into the upper bank of the cingulate sulcus, i.e., the CMA), the cortices lining the left anterior part of the intraparietal sulcus and the bilateral postcentral sulcus, and bilateral cerebellar hemispheres [lobule VI according to Schmahmann et al. (1999)] (Figs. 2, Table 2).
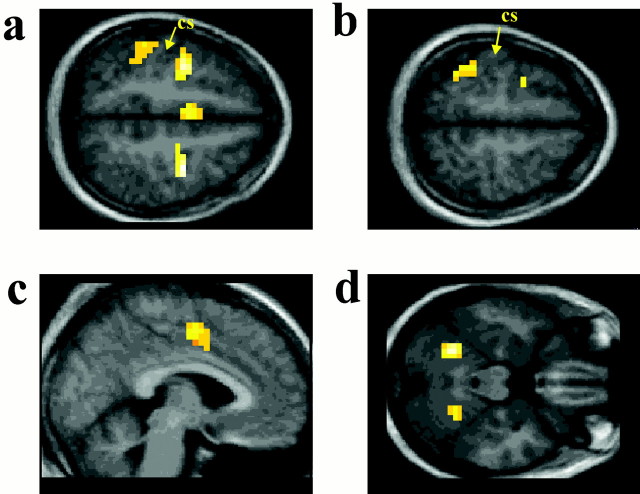
Fig. 2.
The nonsynergistic movement task is contrasted with the synergistic movement task. Increased activity is shown during the nonsynergistic movement present in the bilateral PMD and the cortex lining the left postcentral sulcus (a), the cortex lining the left anterior intraparietal sulcus (b), the SMA/CMA (c), and the bilateral cerebellar hemispheres (lobule VI) (d). The sections correspond to the following Talaraich coordinates (Talaraich and Tournoux, 1988): a,z = +52; b, z = +60;c, x = −4; d, z= −24. Activations (yellow; p < 0.05 after correction for the number of multiple comparisons) are superimposed on a mean anatomical MRI from the eight subjects.cs, Central sulcus. (The activation of the right postcentral cortex is not visible on these sections.)
Both movement tasks activated a similar set of brain regions in comparison with the resting baseline condition: left M1, left primary somatosensory cortex (S1), left PMD, bilateral SMA and CMA, the cortices lining the anterior part of the left intraparietal sulcus, the bilateral parietal operculum, the bilateral cortices lining the lateral fissure (the cluster overlapping with the inferior frontal and superior temporal cortices), the left putamen, the left thalamus, and the bilateral cerebellar hemispheres (all significant at p < 0.05 or better after a correction for multiple comparisons) (Fig.3a–f). Thus, the nonsynergistic coordination of the digits is associated with enhanced activity in several brain regions that also are active during synergistic hand movements. The right PMD and right cortex of the postcentral sulcus were significantly active only during the nonsynergistic digit action.
Fig. 3.
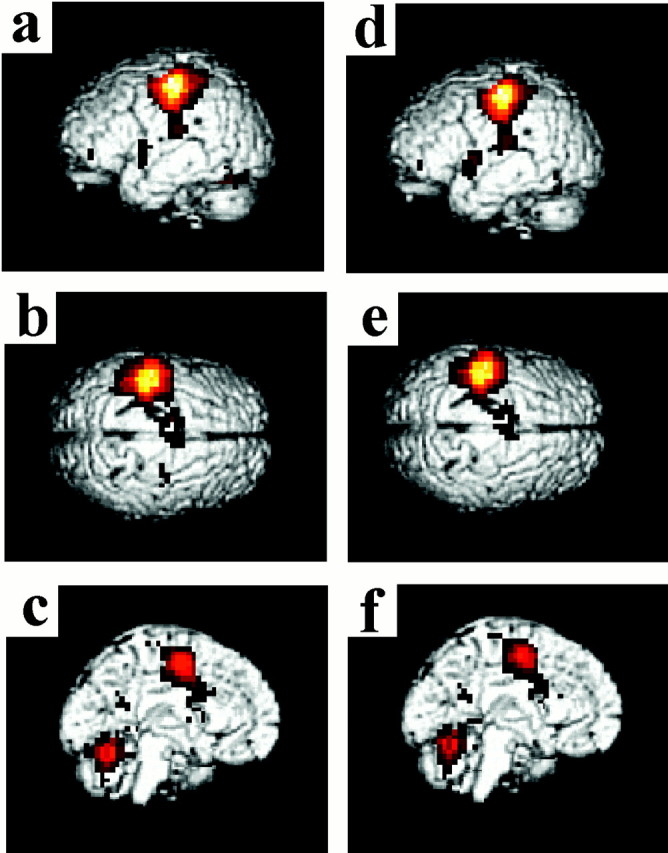
The activations displayed on three-dimensional (3D) reconstructions of the template brain (MNI).a–c, The contrast nonsynergistic digit movement versus rest. d–f, Synergistic digit movements versus rest. The M1/S1 cortex is the area that showed the largest BOLD signal increase when the digit movement tasks were compared with rest (nonsynergistic vs rest: x = −40, y = −24, z = 56;t value, 40.50; synergistic vs rest:x = −40, y = −24,z = 56; t value, 42.19), but there were no significant differences in the degree of activation between the two tasks (p > 0.001 without a correction for multiple comparisons). Additionally, both tasks were associated with significant activations (p < 0.05 corrected) located at the left PMD, SMA/CMA, postcentral sulcus, anterior part of the intraparietal sulcus, and the bilateral parietal operculum, lateral fissure, and lateral cerebellum (the left putamen and thalamus were also active, but this is not shown on these 3D projections of the brain). The top row shows the left hemisphere (a, d), the middle row displays the top view (b, e), and the bottom row highlights the left medial wall (c, f). The activation maps have been thresholded at t = 3.79 for display purposes.
To exclude the eventuality that the lack of difference in the M1 activation between the nonsynergistic and synergistic digit movement was caused by nothing more than the conservative threshold used, we probed the M1/S1 region by using a more liberal statistical criterion. We examined the voxels (using a voxelwise threshold ofp < 0.001 without a correction for the number of multiple comparisons) located in a sphere (diameter 15 mm, corresponding to the “smoothness” of the statistical images) centered around the peak of the activation (x = −40,y = −24, z = 56) obtained from the comparison of the movement tasks with the rest condition. Yet, no voxels in the M1/S1 region showed stronger activity during the nonsynergistic digit movement in comparison to the synergistic ones.
No activations were detected when we contrasted the synergistic versus the nonsynergistic movement, neither when we used the conservative threshold (p > 0.05 corrected) nor when we used the more liberal threshold (p > 0.001, without a correction for the number of multiple comparisons).
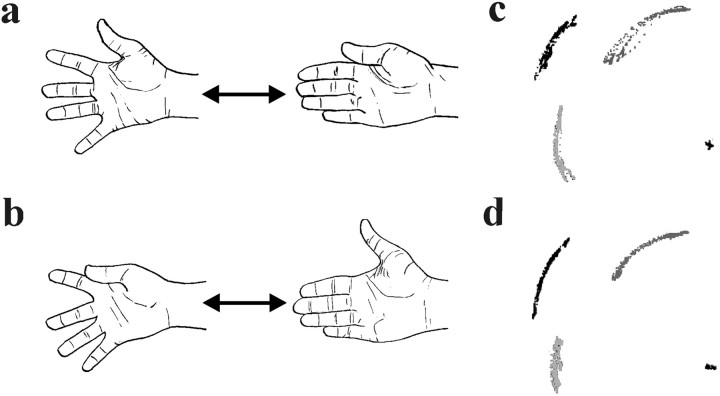
To test whether the increased activation in bilateral PMD, parietal cortex, lateral cerebellum, and the SMA/CMA during nonsynergistic digit movement can be generalized, we performed a second experiment that involved abduction and adduction movements of the fingers (i.e., movements that recruited the intrinsic hand muscles instead of the long extrinsic muscles). All digits were spread (i.e., the fingers were abducted and the thumb was extended) and moved together (i.e., the fingers were adducted and the thumb flexed) in “synergistic spreading movement” (Fig.4a,c). This was compared with the “nonsynergistic spreading movement” task when the thumb was moved in opposite direction to the other digits (when the fingers were abducted, the thumb was flexed, and vice versa) (Fig.4b,d). We used the same training procedures, functional MRI (fMRI) protocols, image processing steps, and statistical analysis as in the first experiment (see Materials and Methods). The analysis of the kinematic data showed that there were no significant differences in the amplitude, velocity, or variability of these parameters between the two versions of the finger-spreading movement task (p > 0.05, paired ttest, without correction for multiple comparisons). The brain regions that showed enhanced activity during nonsynergistic flexion–extension movement also showed increased activity when the subjects performed the nonsynergistic spreading movement (all foci in Table 2 showed increases in activity corresponding to p < 0.05 at each voxel after a correction for multiple comparisons). Thus, the activation of these areas reflects the nonsynergistic movement pattern, regardless of the muscles used to move the digits.
Fig. 4.
The tasks used in the complementary experiment. a, Synergistic spreading movement.b, Nonsynergistic spreading movement (the right hand is used). c and d display a recording of the movement trajectories of the thumb (dark gray), the index finger (black), and the fifth digit (light gray) from one representative subject performing the synergistic movement (c) and the nonsynergistic movement (d) (1/60 sec between each point; scale 1:7).
DISCUSSION
Our results demonstrate that the activity in the bilateral frontal motor areas SMA/CMA and PMD, the bilateral parietal cortex, and the bilateral lateral cerebellum is stronger when the thumb and fingers are moved in a nonsynergistic coordination pattern than when the same digits are moved in a synergistic pattern. These results suggest that the ability of humans to produce skillful hand postures involving independent movements of the digits depends on neural processing in nonprimary frontoparietal areas and the cerebellum.
Several potentially confounding factors can be excluded. The number and amplitude of the movements of the digits in the nonsynergistic and synergistic tasks were the same. The thumb can be moved relative to the other digits with little anatomical constraints (Napier, 1961;Häger-Ross and Schieber, 2000), and the movements were performed at a slow pace to minimize any putative passive biomechanical coupling between the digits. The subjects experienced the movements as simple and performed them as requested in both tasks without mistakes. In both tasks the subjects alternated between two hand postures, which means that the sequential organization of the movement were the same in the two tasks. Thus, differences in the number and amplitude of movements, the biomechanical constraints, the general task difficulty, or the sequential organization of the task could not explain the results. This means that the different functional activation associated with the two tasks reflects the difference in the central representation of the two movement patterns.
The stronger BOLD signals observed in the frontal, parietal, and cerebellar regions during the nonsynergistic digit movement in comparison with the synergistic ones demonstrates that the execution of the nonsynergistic hand postures involves more synaptic activity in these centers (Logothetis et al., 2001). The nonsynergistic movement differs from the synergistic ones in two important aspects: in the coordination pattern per se and in that the thumb is moved independently in relation to the other digits. During the synergistic mode, all digits flex and extend together in an innate coordination pattern already present in its reflex form at birth. In contrast, nonsynergistic coordination patterns have to be learned by practice. Thus, the strong activation of the nonprimary areas during the nonsynergistic movement tasks could reflect learned motor representations mediating the coordination of the movements of the thumb and the four fingers (Ioffe, 1992).
The increased activity associated with the nonsynergistic patterns could also reflect sensorimotor mechanisms needed for the individualization of the movements of the thumb relative to the four fingers. From a motor control perspective, independent movements of digits involve higher degrees of freedom to be controlled than movements in which the digits are synergistically coupled (Napier, 1961; Bernstein, 1967). It seems reasonable to assume that higher degrees of freedom will increase the demands on the sensorimotor processing in the cortical networks because additional elements of the motor apparatus have to be explicitly controlled. Thus, the increased activation associated with the nonsynergistic movement could reflect neural activity specifically related to the relatively independent mode of control of the thumb movements in relation to the fingers.
The M1 and S1 were strongly activated both when the nonsynergistic hand postures were generated and when the synergistic ones were produced, but the level of activity was similar in these two tasks (see Results for details). The absence of further increases in M1 during the nonsynergistic movement does not mean that certain subpopulations of neurons in M1 are not critically involved in the production of independent movements of the digits. Indeed, there is strong evidence that the descending output signals from the corticospinal neurons in M1 are important for the generation of independent finger movements (Muir and Lemon, 1983; Porter and Lemon, 1993; Bennett and Lemon, 1996;Rouiller et al., 1998). The descending efferent signals responsible for the fractionation of the muscle activity are believed to involve sophisticated patterns of excitatory and inhibitory signals to the finger muscles and active suppression of the innate synergistic motor pattern (Ioffe, 1992; Schieber, 1996). Our results showing that the BOLD contrast signal in M1 is similar during nonsynergistic and synergistic digit movement are not incompatible with the results from the single-cell recordings. The BOLD signal is a reliable index of brain activity that corresponds well to the overall level of synaptic activity in an area, but it may be a less reliable index for the spiking activity of the output neurons (Turner et al., 1997; Logothetis et al., 2001). It is possible that an increased discharge rate of small groups of corticospinal neurons belonging to a larger zone of active M1 cortex is not associated with detectable increases in the BOLD signal.
The increased activation of the SMA/CMA and PMD during the generation of nonsynergistic hand postures reflects an increased overall synaptic activity in these areas, specifically when the thumb and fingers are moved in a nonsynergistic pattern. This synaptic activity probably reflects the processing of motor and somatosensory signals in the local neural networks in these areas and corticocortical transformation of motor-related information between these centers (which are interconnected in other primates). The motor-related signals in the SMA/CMA and PMD can be transformed into muscular commands for the fingers and thumb either directly via corticospinal neurons in these areas (Dum and Strick, 1991, 1996; He et al., 1993) or indirectly via corticocortical connections to the corticospinal neurons of the hand section of M1 (Pandya and Vignolo, 1971; Tokuno and Tanji, 1993).
Increased activation in the parietal areas (the anterior part of the left intraparietal sulcus and the bilateral postcentral sulcus) may indicate increased processing related to the planning of the movement trajectories of the digits and the somatosensory guidance of the digit movements (Mountcastle et al., 1975; Iwamura and Tanaka, 1991; Gardner et al., 1999). Lesions in the monkey postcentral sulcus and the anterior part of the intraparietal sulcus lead to clumsy finger movements and poor coordination of the digits (Iwamura and Tanaka, 1991; Gallese et al., 1994). The parietal areas and the SMA/CMA and PMD are reciprocally connected, forming distributed frontoparietal networks (Wise et al., 1997; Rizzolatti et al., 1998). Together with the activity detected in the SMA/CMA and PMD, these parietal activations might provide a neurophysiological explanation for the classical neurological observation that focal lesions in these frontoparietal regions cause a certain type of apraxia, specifically impairing hand dexterity (known as innervatory/limb-kinetic apraxia) (Kleist, 1907;Luria, 1966; Freund, 1987; Leiguarda and Marsden, 2000).
The increased cerebellar activity posterior to the classical motor section of the anterior cerebellar lobule corresponds well to the results of earlier studies showing impairments in the coordination of skillful finger, hand, and arm movements in human subjects and monkeys with damage to the lateral cerebellum (Holmes, 1939; Dow, 1987; Thach et al., 1992; Muller and Dichgans, 1994). This section of the cerebellar hemisphere (lobule VI) is probably interconnected with the SMA (and perhaps the PMD) and the posterior parietal cortex (Schmahmann, 1996, 2000). Thus, the cerebellum is part of a distributed network controlling skillful movement of the digits.
The present fMRI result, suggesting a role for the SMA/CMA, the PMD, the cortices of the intraparietal and the postcentral sulci, and the lateral cerebellum in the control of nonsynergistic movement of the digits is novel. Increases in the activity of nonprimary frontoparietal areas and the cerebellum have been reported previously during various skillful hand actions involving independent movements of the digits, when the effects of the muscular contractions were eliminated by including appropriate control tasks, such as object manipulation using the fingertips (Binkofski et al., 1999; Ehrsson et al., 2000, 2001;Kuhtz-Buschbeck et al., 2001), finger–thumb opposition sequences (Roland et al., 1980; Sadato et al., 1996), handwriting (Rijntjes et al., 1999), and bimanual movements of digits (Sadato et al., 1997;Stephan et al., 1999). However, in these experiments, the activations could be explained by factors other than the control of nonsynergistic movements and postures of the digits, such as sensorimotor integration (e.g., the utilization of tactile signals for fingertip force control during dexterous manipulation), cognitive demands (e.g., retaining the memory of a motor sequence), and bimanual coordination.
In conclusion, our results suggest that a distributed network including nonprimary motor (SMA/CMA and PMD), parietal, and cerebellar regions is critically involved in the control of skillful movements of the digits. The role of the corticospinal neurons in M1 (and possibly other areas) is to transform the motor commands from this nonprimary network into descending efferent signals to the digit muscles. The distributed nature of this motor representation explains why injuries in any of these nonprimary regions impair dexterity in primates.
Footnotes
This work was supported by the Axel & Margaret Ax:sson Johnson Foundation and the Swedish Research Council (14X-5925). We thank Prof. Per E. Roland, Prof. Roland S. Johansson, and Dr. Fredrik Ullén for valuable comments on this manuscript.
Correspondence to should be addressed to H. Henrik Ehrsson, Motoriklab, Department of Woman and Child Health, Astrid Lindgren Children's Hospital, Stockholm, Sweden, SE-171 76. E-mail:Henrik.Ehrsson@neuro.ki.se.
REFERENCES
- 1.Ashburner J, Friston KJ. Spatial transformation of images. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. Academic; London: 1997. pp. 43–58. [Google Scholar]
- 2.Bennett KM, Lemon RN. Corticomotoneuronal contribution to the fractionation of muscle activity during precision grip in the monkey. J Neurophysiol. 1996;75:1826–1842. doi: 10.1152/jn.1996.75.5.1826. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein N. The co-ordination and regulation of movements. Pergamon; Oxford: 1967. [Google Scholar]
- 4.Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H-J. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- 5.Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol. 1991;65:1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- 6.Dow RS. Cerebellum, pathology: symptoms and signs. In: Adelman G, editor. Encyclopedia of neuroscience. Birkhauser; Boston: 1987. pp. 203–206. [Google Scholar]
- 7.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision versus power grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- 10.Ehrsson HH, Fagergren A, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI Study. J Neurophysiol. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- 11.Evans AC, Kamber M, Collins DL, Macdonald D. An MRI-based probablistic atlas of neuroanatomy. In: Shorvon S, Fish D, Andermann F, Bydder GM, Stefan H, editors. Magnetic resonance scanning and epilepsy. Plenum; New York: 1994. pp. 263–274. [Google Scholar]
- 12.Freund H-J. Abnormalities of motor behavior after cortical lesions in humans. In: Brooks V, editor. Handbook of physiology, Sect 1, The nervous system, Part 2. American Physiological Society; Bethesda, MD: 1987. pp. 763–810. [Google Scholar]
- 13.Friston KJ, Holms A, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 14.Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. NeuroReport. 1994;5:1525–1529. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Gardner EP, Ro JY, Debowy D, Ghosh S. Facilitation of neuronal activity in somatosensory and posterior parietal cortex during prehension. Exp Brain Res. 1999;127:329–354. doi: 10.1007/s002210050803. [DOI] [PubMed] [Google Scholar]
- 16.Halverson HM. Study of prehension in infants. Genet Psychol Mon. 1931;10:110–286. [Google Scholar]
- 17.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes A, Poline JB, Friston KJ. Characterising brain images with the general linear model. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. Academic; London: 1997. pp. 59–84. [Google Scholar]
- 19.Holmes G. The cerebellum of man. The Hughling Jackson Memorial Lecture. Brain. 1939;62:1–30. [Google Scholar]
- 20.Häger-Ross C, Schieber MH. Quantifying the independence of human finger movements: comparisons of digits, hands, and movement frequencies. J Neurosci. 2000;15:8542–8550. doi: 10.1523/JNEUROSCI.20-22-08542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioffe ME. Mechanisms of motoric learning. Neurosci Behav Physiol. 1992;22:481–494. doi: 10.1007/BF01185438. [DOI] [PubMed] [Google Scholar]
- 22.Iwamura Y, Tanaka M. Organization of the first somatosensory cortex for manipulation of objects: an analysis of behavioral changes induced by muscimol injection into identified cortical loci of awake monkeys. In: Franzen O, Westman J, editors. Information processing in the somatosensory system. Macmillan; London: 1991. pp. 371–380. [Google Scholar]
- 23.Kleist K. Corticale (innervatorische) apraxie. Jahrbuch Psychiatrie Neurologie. 1907;28:46–112. [Google Scholar]
- 24.Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI Study. Eur J Neurosci. 2001;14:382–390. doi: 10.1046/j.0953-816x.2001.01639.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuypers HG. Anatomy of the descending pathways. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology—the nervous system. American Physiological Society; Bethesda, MD: 1981. pp. 597–666. [Google Scholar]
- 26.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Leiguarda RC, Marsden CD. Limb apraxias: higher-order disorders of sensorimotor integration. Brain. 2000;123:860–879. doi: 10.1093/brain/123.5.860. [DOI] [PubMed] [Google Scholar]
- 28.Lemon RN, Baker SN, Davis JA, Kirkwood PA, Maier MA, Yang HS. The importance of the cortico-motoneuronal system for control of grasp. Novartis Found Symp. 1998;218:202–215. doi: 10.1002/9780470515563.ch11. [DOI] [PubMed] [Google Scholar]
- 29.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the BOLD signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 30.Luria AR. Higher cortical functions in man. Basic Books; New York: 1966. [Google Scholar]
- 31.Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- 32.Muir RB, Lemon RN. Corticospinal neurons with a special role in precision grip. Brain Res. 1983;261:312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- 33.Muller F, Dichgans J. Dyscoordination of pinch and lift forces during grasp in patients with cerebellar lesions. Exp Brain Res. 1994;101:485–492. doi: 10.1007/BF00227341. [DOI] [PubMed] [Google Scholar]
- 34.Napier JRJ. The prehensile movements of the human hand. J Bone Joint Surg. 1956;38B:902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- 35.Napier JRJ. Prehensility and opposability in the hands of primates. Symp Zool Soc Lond. 1961;5:115–132. [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Olesen J. Contralateral focal increase of cerebral blood flow in man during arm work. Brain. 1971;94:635–646. doi: 10.1093/brain/94.4.635. [DOI] [PubMed] [Google Scholar]
- 38.Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Res. 1971;26:217–233. [PubMed] [Google Scholar]
- 39.Passingham RE. The frontal lobes and voluntary action. Oxford UP; Oxford: 1993. [Google Scholar]
- 40.Passingham RE, Perry VH, Wilkinson F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain. 1983;106:675–705. doi: 10.1093/brain/106.3.675. [DOI] [PubMed] [Google Scholar]
- 41.Poline JB, Holmes A, Worsley K, Friston KJ. Making statistical inferences. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. Academic; London: 1997. pp. 85–106. [Google Scholar]
- 42.Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford UP; New York: 1993. [Google Scholar]
- 43.Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RSJ, Weiller C. A blueprint for movement: functional and anatomical representations in the human motor system. J Neurosci. 1999;19:8043–8048. doi: 10.1523/JNEUROSCI.19-18-08043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 45.Roland PE. Brain activation. Wiley; New York: 1993. Motor functions. pp. 237–267. [Google Scholar]
- 46.Roland PE, Zilles K. Functions and structures of the motor cortices in humans. Curr Opin Neurobiol. 1996;6:773–781. doi: 10.1016/s0959-4388(96)80027-4. [DOI] [PubMed] [Google Scholar]
- 47.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 48.Rouiller EM, Yu XH, Moret V, Tempini A, Wiesendanger M, Liang F. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion. Eur J Neurosci. 1998;10:729–740. doi: 10.1046/j.1460-9568.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 49.Sadato N, Campbell G, Ibanez V, Deiber M, Hallett M. Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci. 1996;16:2691–2700. doi: 10.1523/JNEUROSCI.16-08-02691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci. 1997;17:9667–9674. doi: 10.1523/JNEUROSCI.17-24-09667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schieber MH. Individuated finger movements: rejecting the labeled-line hypothesis. In: Wing AM, Haggard P, Flanagan JR, editors. Hand and brain. Academic; New York: 1996. pp. 81–98. [Google Scholar]
- 52.Schmahmann JD. From movement to thought: anatomical substrates of the cerebellar contribution of cognitive processing. Hum Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 53.Schmahmann JD. Cerebellum and brain stem. In: Toga AW, Mazziotta JC, editors. Brain mapping: the systems. Academic; San Diego: 2000. [Google Scholar]
- 54.Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- 55.Stephan KM, Binkofski F, Posse S, Seitz RJ, Freund H-J. Cerebral midline structures in bimanual coordination. Exp Brain Res. 1999;128:243–249. doi: 10.1007/s002210050844. [DOI] [PubMed] [Google Scholar]
- 56.Talaraich J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- 57.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 58.Tokuno H, Tanji J. Input organization of distal and proximal forelimb areas in the monkey primary motor cortex: a retrograde double labeling study. J Comp Neurol. 1993;333:199–209. doi: 10.1002/cne.903330206. [DOI] [PubMed] [Google Scholar]
- 59.Turner R, Howseman A, Rees G, Josephs O. Functional imaging with magnetic resonance imaging. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. Academic; London: 1997. pp. 467–486. [Google Scholar]
- 60.Twitchell T. Reflex mechanisms and the development of prehension. In: Connolly K, editor. Mechanisms of motor skill development. Academic; New York: 1970. pp. 25–45. [Google Scholar]
- 61.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Ann Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]