Fig. 2.
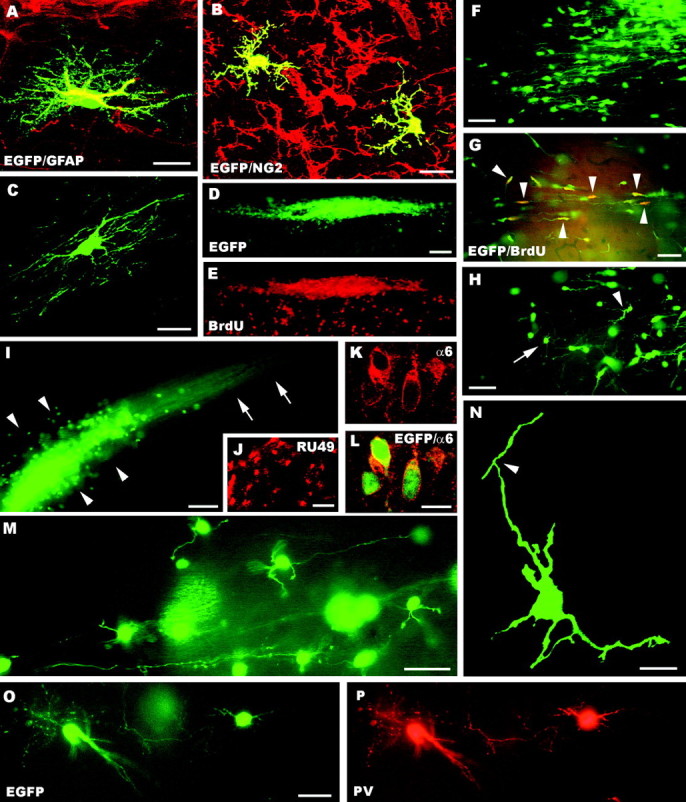
A–P, Fate of postnatal cerebellar cells engrafted in ectopic regions of the embryonic brainin utero. The confocal pictures A–C show glial phenotypes generated by postnatal cerebellar precursors, including GFAP-positive astrocytes (A), NG2-positive immature oligodendrocytes (B, taken from an animal killed at P1), and mature oligodendrocytes properly integrated in host white matter tracts (C). A large aggregate of small, densely packed transplanted cells is shown inD (taken from an animal killed at P1); BrdU labeling of the same microscopic field (E) reveals the intense proliferative activity of these cells (numerous host cells outside the cluster are also labeled). Migrating cells, with typical leading and trailing processes, radiate from such clusters (F) and likely continue to divide, as shown by BrdU incorporation (G, arrowheads point to some double-labeled cells). At the end of their migratory phase (H), the cells show several short processes radiating from the perikaryon (e.g.,arrowhead) and eventually acquire the typical morphology of granule cells (e.g., arrow). Mature granule cells can be found scattered or clustered in aggregates (arrowheads in I) with bundles of parallel fibers (arrows). The identification of these cells is further supported by the expression of RU49 mRNA (J) as well as α6 subunit of the GABAA receptor (K, L). The typical morphology of granule neurons, with small round cell bodies, a few short clawed dendrites and thin varicose axons, is displayed inM. In addition, the confocal image Nshows an immature granule cell bearing the characteristic T-shaped bifurcation (arrowhead) of the parallel fiber.O and P display parvalbumin-immunopositive molecular layer interneurons.GFAP, Glial fibrillary acidic protein; α6, α6 subunit of the GABAA receptor; BrdU, bromodeoxyuridine; PV, parvalbumin; EGFP, enhanced green fluorescent protein. Scale bars: A, C, K, L, N, 10 μm; B, 15 μm; M, O, P, 30 μm; F–H, J, 50 μm; D, E, I, 100 μm.
