Fig. 7.
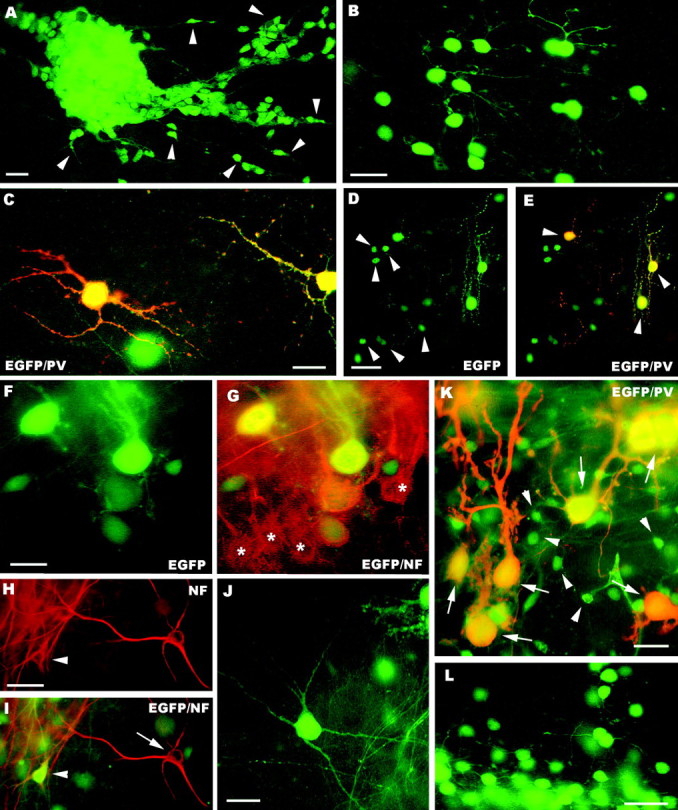
A–H, Fate of postnatal (A–E) and embryonic (F–L) cerebellar cells transplanted to cerebellar (A–C, F–J) or neocortical explants (D, E, K, L). In the cerebellar explants, postnatal cerebellar progenitors form dense aggregates (A) with numerous migrating elements (some are pointed byarrowheads). Mature granule cells, bearing typical morphological features, are shown in B, whereas parvalbumin-immunopositive molecular layer interneurons are displayed in C. D and E illustrate the neuronal phenotypes generated by postnatal cerebellar cells grafted to neocortical explants, granule cells (arrowheads in D) and molecular layer interneurons (arrowheadsin E). The latter are also labeled by anti-parvalbumin antibodies (E). The micrographs Fand G show EGFP-positive Purkinje cells derived from embryonic precursors intermingled with their host counterparts (asterisks in G) in the cerebellar cortex of the explant. Examples of EGFP-positive multipolar neurons in cerebellar explants are illustrated in H–J. Some of these neurons (arrowhead in H andI) are localized in the deep nuclei of the explant, and can be stained by anti-neurofilament antibodies (arrow in I points to an immunolabeled host neuron). In neocortical explants embryonic cerebellar progenitors also generate parvalbumin-immunopositive Purkinje cells (arrows in K) with variably shaped dendritic trees and granule cells (K, L, some are pointed by arrowheads in K).PV, Parvalbumin; NF, neurofilament;EGFP, enhanced green fluorescent protein. Scale bars:B, C, 20 μm; A, F, G, K, 25 μm;L, 30 μm; D, E, H–J, 50 μm.
